Traumatic Brain Injury
Traumatic brain injury (TBI) is common, and a significant cause of morbidity and mortality.
There is also a significant weighting towards younger patients, with the subsequent impact on society for patients who survive and may be left with varying degrees of disability and dependence.
This has led to a notable public health push to prevent such injuries occurring, because as we shall discuss, the medical input following a TBI has only a relatively small role to play in reducing these outcomes.
There is also a significant weighting towards younger patients, with the subsequent impact on society for patients who survive and may be left with varying degrees of disability and dependence.
This has led to a notable public health push to prevent such injuries occurring, because as we shall discuss, the medical input following a TBI has only a relatively small role to play in reducing these outcomes.
Pathophysiology
The key concept here is the differentiation between primary injury and secondary injury.
The primary injury refers to the damage to neuronal tissue that is done at the time of impact.
The secondary injury refers to neuronal injury that occurs as a result of the many effects that occur as a result of the TBI or other injuries e.g. hypoxia from unconsciousness.
The two main forms that brain injuries come in are penetrating injuries and non-penetrating injuries.
Penetrating brain injuries are less common, and much of the discussion here is about non-penetrating injuries, although many of the principles overlap.
The primary injury is said to occur at the time of impact.
TBI involves transmission of force to the head and brain. This force (and energy) can arise from a plethora of sources (kinetic energy of a moving car, potential energy of a body up a ladder) and will be transmitted to the brain tissues in a similarly diverse array of ways.
This energy is applied to the neuronal, glial and vascular tissues of the brain and results in shearing and compression forces.
This produces a cascade of inflammatory and neurotoxic pathways
The secondary injury arises from a delayed physiological insult upon this already vulnerable neural tissue.
The primary modality is from ischaemia, arising from complications such as hypotension, raised intracranial pressure, hypoxia.
One main focus of the management the patient with TBI is to prevent these secondary injuries from occurring.
This differentiation is a little simplistic, as it is being recognised that some ‘primary injury’ continues for a period of time after the actual impact, whilst secondary injury processes may begin to occur nearly straight away.
However, this description is useful for guiding the management of patients.
A wide range of specific intracranial injuries may occur following a head trauma:
The primary injury refers to the damage to neuronal tissue that is done at the time of impact.
The secondary injury refers to neuronal injury that occurs as a result of the many effects that occur as a result of the TBI or other injuries e.g. hypoxia from unconsciousness.
The two main forms that brain injuries come in are penetrating injuries and non-penetrating injuries.
Penetrating brain injuries are less common, and much of the discussion here is about non-penetrating injuries, although many of the principles overlap.
The primary injury is said to occur at the time of impact.
TBI involves transmission of force to the head and brain. This force (and energy) can arise from a plethora of sources (kinetic energy of a moving car, potential energy of a body up a ladder) and will be transmitted to the brain tissues in a similarly diverse array of ways.
This energy is applied to the neuronal, glial and vascular tissues of the brain and results in shearing and compression forces.
This produces a cascade of inflammatory and neurotoxic pathways
The secondary injury arises from a delayed physiological insult upon this already vulnerable neural tissue.
The primary modality is from ischaemia, arising from complications such as hypotension, raised intracranial pressure, hypoxia.
One main focus of the management the patient with TBI is to prevent these secondary injuries from occurring.
This differentiation is a little simplistic, as it is being recognised that some ‘primary injury’ continues for a period of time after the actual impact, whilst secondary injury processes may begin to occur nearly straight away.
However, this description is useful for guiding the management of patients.
A wide range of specific intracranial injuries may occur following a head trauma:
- Diffuse axonal injury
- Contusions
- Subarachnoid haemorrhage
- Extra-dural haemorrhage - often arterial in origin, commonly the middle meningeal artery
- Sub-dural haemorrhage - usually from tearing of the veins as they penetrate the dura
- Skull fractures
Presentation
The number of ways that TBI can present is very wide, but it is important to gain as much information as possible about the nature of the trauma, as this will help guide management of the TBI, and other associated injuries.
Some aspect of the history also have an impact on the risk assessment and prognostication of the patient’s injuries.
This list isn’t exhaustive but enquire about:
Mechanism of Injury
Fall: Height fallen (number of steps if stairs), surface landed on, duration of time prior to help, reason for fall (syncope),
Motor vehicle collision: Role of patient (pedestrian, driver, passenger), wearing seatbelt, airbag deployed, fate of other car occupants, evidence of projection (ejected from car, bulls-eyed windscreen), estimated speed.
Mobilisation post injury?
Extraction time.
Initial observations
GCS, heart rate, O2 saturations, blood pressure, temperature, capillary glucose.
Past medical history
Any significant co-morbidities.
Specific enquiry into anticoagulants or antiplatelet agents.
Some aspect of the history also have an impact on the risk assessment and prognostication of the patient’s injuries.
This list isn’t exhaustive but enquire about:
Mechanism of Injury
Fall: Height fallen (number of steps if stairs), surface landed on, duration of time prior to help, reason for fall (syncope),
Motor vehicle collision: Role of patient (pedestrian, driver, passenger), wearing seatbelt, airbag deployed, fate of other car occupants, evidence of projection (ejected from car, bulls-eyed windscreen), estimated speed.
Mobilisation post injury?
Extraction time.
Initial observations
GCS, heart rate, O2 saturations, blood pressure, temperature, capillary glucose.
Past medical history
Any significant co-morbidities.
Specific enquiry into anticoagulants or antiplatelet agents.
Assessment
The initial assessment/treatment will be part of a major trauma presentation, and will rarely be identified as a purely isolated head injury pre-hospital.
As such, the initial assessment will be as per any other major trauma presentation.
The National Institute for Health and Care Excellence (NICE) recommends that a systematic approach is taken in these patients to optimise their care.
A number of groups provide guidance on a structured approach, such as the Advanced Trauma Life Support Group (ATLS).
As with other acutely ill patients it essentially involves a A to E assessment and treating life threatening complications as they are identified.
This initial assessment/resuscitation process is discussed elsewhere.
In patients with suspected TBI, the neurological component of the assessment is particularly important as it can provide many essential signs and guide later management.
At a minimum this should include:
As such, the initial assessment will be as per any other major trauma presentation.
The National Institute for Health and Care Excellence (NICE) recommends that a systematic approach is taken in these patients to optimise their care.
A number of groups provide guidance on a structured approach, such as the Advanced Trauma Life Support Group (ATLS).
As with other acutely ill patients it essentially involves a A to E assessment and treating life threatening complications as they are identified.
This initial assessment/resuscitation process is discussed elsewhere.
In patients with suspected TBI, the neurological component of the assessment is particularly important as it can provide many essential signs and guide later management.
At a minimum this should include:
- GCS, with clear documentation of the best motor score
- Pupil assessment
- Gross assessment for focal neurology
Imaging
The aspects of assessment specific to TBI (other than those in the A to E assessment) relate to imaging of the head +/- the cervical spine.
NICE provide some very useful guidance on the indications for imaging in these patients (https://www.nice.org.uk/guidance/cg176/chapter/1-recommendations)
Essentially, CT scan is the imaging of choice because of its logistical ease compared to MRI scanning.
However, it is recognised that MRI does have greater sensitivity for some pathology, and may be indicated in specific conditions.
NICE classify the need for a CT head, and it’s urgency, based on the presence of clinical risk factors.
An urgent (within 1 hour) CT head is needed if the following features are present:
An expedited (within 8 hours) CT head scan is needed if the following risk factors are present:
NICE provide some very useful guidance on the indications for imaging in these patients (https://www.nice.org.uk/guidance/cg176/chapter/1-recommendations)
Essentially, CT scan is the imaging of choice because of its logistical ease compared to MRI scanning.
However, it is recognised that MRI does have greater sensitivity for some pathology, and may be indicated in specific conditions.
NICE classify the need for a CT head, and it’s urgency, based on the presence of clinical risk factors.
An urgent (within 1 hour) CT head is needed if the following features are present:
- GCS <13 at ED assessment
- GCS <15 at 2 hours after the initial assessment
- Signs of a basal skull fracture
- Suspected open or depressed skull fracture
- Post-traumatic seizure
- Focal neurological deficit
- More than one episode of vomiting
An expedited (within 8 hours) CT head scan is needed if the following risk factors are present:
- The patient is on an anticoagulant
- The patient has experienced a LOC or some PTA and:
- Age 65 or older
- Has a history of bleeding or clotting disorders
- Has a dangerous mechanism of impact (pedestrian vs car, fall from height over 1 metre, ejected from a motor vehicle).
- More than 30 mins retrograde amnesia of events before the trauma
- Age 65 or older
Severity
The initial assessment of severity can be a little difficult due to the wide variation in presentation, but there are several domains that can be assessed to help with this.
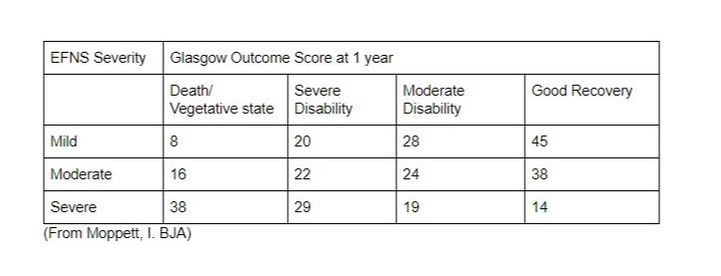
The European Federation of Neurosurgeons (EFNS) categorised the severity of the TBI based on the admission GCS and other clinical signs.
Mild: GCS 13-15
Moderate: GCS 9-12
Severe: GCS 8 or less
The ‘Mild’ group can be further subdivided:
Category 0: GCS 15, no loss of consciousness (LOC), no post traumatic amnesia (PTA), no risk factors
Category 1: GCS 15, LOC <30mins, PTA <1hour, no risk factors.
Category 2: GCS 15, risk factors present
Category 3: GCS 13-14.
The motor component of the GCS is also an important marker of severity, and a prognostic marker.
There are other risk factors which are associated with a poor outcome.
When considering the mechanism of injury these are:
When considering examination findings:
When considering CT findings, some findings are associated with a bad outcome:
The European Federation of Neurosurgeons (EFNS) categorised the severity of the TBI based on the admission GCS and other clinical signs.
Mild: GCS 13-15
Moderate: GCS 9-12
Severe: GCS 8 or less
The ‘Mild’ group can be further subdivided:
Category 0: GCS 15, no loss of consciousness (LOC), no post traumatic amnesia (PTA), no risk factors
Category 1: GCS 15, LOC <30mins, PTA <1hour, no risk factors.
Category 2: GCS 15, risk factors present
Category 3: GCS 13-14.
The motor component of the GCS is also an important marker of severity, and a prognostic marker.
There are other risk factors which are associated with a poor outcome.
When considering the mechanism of injury these are:
- Penetrating brain injury (compared to blunt)
- Pedestrians in RTCs
- Age - progressive increase in risk with age
- Female - women appear to do less well compared to men
When considering examination findings:
- GCS - as noted aboved
- Pupils - bilateral fixed dilated pupils have a poor outcome in 70-90% of cases. Unilateral blown pupil is not as bad, but worse than if bilaterally reactive pupils, when the rate of a poor outcome is around 30%.
When considering CT findings, some findings are associated with a bad outcome:
- Traumatic SAH
- Mid-line shift
- Effacement of the basal cisterns
Prognostication
The outcome following TBI has several impacting factors, but one important factor is the GCS at presentation:
However, prognostication is very difficult in these patients, even up to 1 month after the injury.
The CRASH group have prodiuced an online tool that can help to some degree with this: http://www.trialscoordinatingcentre.lshtm.ac.uk/Risk%20calculator/index.html
The CRASH group have prodiuced an online tool that can help to some degree with this: http://www.trialscoordinatingcentre.lshtm.ac.uk/Risk%20calculator/index.html
Management
Initial Resuscitation
As noted earlier, the approach to the patient with TBI requires the systematic approach applicable to all critically ill patients.
An A to E approach is particularly important in these patients because of the impact of impaired physiology on secondary brain injury.
Airway and Breathing (Including C-spine precautions)
Severe TBI often has a deleterious impact on respiratory function and airway protection.
This will often need intervention to optimise gas exchange and provide airway protection.
Some important points are:
Circulation
Cardiovascular compromise has a similarly deleterious effect on outcome in TBI.
When TBI is part of polytrauma, such knowledge must be balanced against other aspect of the patient’s care e.g. permissive hypotension when bleeding.
Important points include:
Disability and Exposure
Sedation is required to tolerate aggressive intervention such as intubation and ventilation.
Inadequate sedation can raise ICP, although the deleterious cardiovascular effects must also be considered.
As already noted, other injuries must be actively considered and sought for. They occur in 50% of patients with severe TBI.
Monitor the patient’s blood sugar, targeting a range of 6-10 mmol/L.
An A to E approach is particularly important in these patients because of the impact of impaired physiology on secondary brain injury.
Airway and Breathing (Including C-spine precautions)
Severe TBI often has a deleterious impact on respiratory function and airway protection.
This will often need intervention to optimise gas exchange and provide airway protection.
Some important points are:
- Any time spent with an SpO2 below 90% increases mortality.
- Time spent with an SpO2 below 60% results in a 100% rate of mortality or severe diability
- Because of the mechanism of injury, cervical spine injury is relatively common in cases of severe TBI - its presence should be assumed.
Circulation
Cardiovascular compromise has a similarly deleterious effect on outcome in TBI.
When TBI is part of polytrauma, such knowledge must be balanced against other aspect of the patient’s care e.g. permissive hypotension when bleeding.
Important points include:
- A single episode of BP below 90 mmHg (mean duration 9 minutes) doubles mortality.
- Two episodes results in an eightfold increase in mortality!
Disability and Exposure
Sedation is required to tolerate aggressive intervention such as intubation and ventilation.
Inadequate sedation can raise ICP, although the deleterious cardiovascular effects must also be considered.
As already noted, other injuries must be actively considered and sought for. They occur in 50% of patients with severe TBI.
Monitor the patient’s blood sugar, targeting a range of 6-10 mmol/L.
ICP Monitoring
Part of the monitoring of patients with TBI involves monitoring of ICP.
The concept of this is based on the recognised impact that raised ICP has on impaired perfusion of brain tissue, and the risk of herniation of brain across compartments.
Indeed, it is clear that elevations in ICP result in worse outcomes.
As such the approach to using ICP monitoring has generally not been extensively investigated because of the lack of equipoise.
However, there is some evidence from a South American RCT that the use of ICP monitoring doesn’t apply an outcome benefit compared to combined clinical and radiological assessment.
However, there is other study evidence of improved short-term outcome with invasive ICP monitoring, although not RCT standard.
Current recommendation from the Brain Trauma Foundation is that patients with severe TBI should be managed with using information from ICP monitoring.
The concept of this is based on the recognised impact that raised ICP has on impaired perfusion of brain tissue, and the risk of herniation of brain across compartments.
Indeed, it is clear that elevations in ICP result in worse outcomes.
As such the approach to using ICP monitoring has generally not been extensively investigated because of the lack of equipoise.
However, there is some evidence from a South American RCT that the use of ICP monitoring doesn’t apply an outcome benefit compared to combined clinical and radiological assessment.
However, there is other study evidence of improved short-term outcome with invasive ICP monitoring, although not RCT standard.
Current recommendation from the Brain Trauma Foundation is that patients with severe TBI should be managed with using information from ICP monitoring.
Subsequent Management
Much of the subsequent management of TBI focuses on the control of ICP, although there is a significant degree of neurosurgical expertise involved in some pathologies.
ICP as a topic is covered elsewhere, including a review of the physiology.
A tiered approach is used, and a good example is that used by SRFT, available at: http://www.neuroicu.guru/
Whenever there is a deterioration in the patient’s clinical conditions, the question should be asked as to whether this might be due to an intracranial cause?
If this is possible, repeat imaging of their head may be needed to assess if there is a lesion that is amenable to neurosurgical intervention.
An ICP of > 20 mmHg for over 5 minutes needs an urgent medical review, with an intervention to gain control of the ICP again.
This may involve escalation within the tier, or up to a high tier of management.
Tier 1
This aims to optimise the simple factors of raised ICP.
Tier 2
If tier 1 measures are failing to control the ICP, there is escalation to tier 2.
At this point, there should again be a stop to assess what the problem might be:
Measures include:
Tier 3
At this stage a review is again appropriate to ensure optimal care has been given so far:
At this stage there are limited good options, but they include:
Recent trials have provided challenging data on the implementation of these measures (see RescueICP and Eurotherm) which will be discussed in more detail below..
ICP as a topic is covered elsewhere, including a review of the physiology.
A tiered approach is used, and a good example is that used by SRFT, available at: http://www.neuroicu.guru/
Whenever there is a deterioration in the patient’s clinical conditions, the question should be asked as to whether this might be due to an intracranial cause?
If this is possible, repeat imaging of their head may be needed to assess if there is a lesion that is amenable to neurosurgical intervention.
An ICP of > 20 mmHg for over 5 minutes needs an urgent medical review, with an intervention to gain control of the ICP again.
This may involve escalation within the tier, or up to a high tier of management.
Tier 1
This aims to optimise the simple factors of raised ICP.
- Minimise obstruction to cerebral venous flow - check ETT ties aren’t too tight, removed cervical collars if appropriate (e.g. sedated), neutral head position.
- Head up at 30 degrees - optimises venous drainage
- Ensure normoxia (SpO2 94-98%, pO2 10-12 kPa) - this maintains adequate oxygen delivery to the brain and prevents the vasodilation and rise if cerebral blood volume at low pO2 levels.
- Ventilate to low normocapnia (pCO2 4.5-5 kPa) - the avoids the excessive vasodilation and high blood volumes of higher pCO2 levels, but also minimises the degree of vasoconstriction that might impair CBF.
- Deeply sedate - target a Richmond Agitation and Sedation Score (RASS) of -5. Escalate sedative agents as needed, including adding a benzodiazepine. This reduces cerebral metabolic activity.
- Avoid coughing - this causes significant rises in ICP and so must be avoided, even on deeply stimulating procedures such as suctioning. Neuromuscular blockade may be considered if this isn’t managed with sedation alone.
- Maintain a CPP of 60-70 mmHg - optimise fluid volume status first, but may also need vasopressor therapy in the form of noradrenaline.
- Ensure liberal normoglycaemia - target a glucose of 5-10 mmol/L. This minimises the harm of elevated blood glucose levels and the risks of hypoglycaemic episodes.
- Consider the role for an external ventricular drain (EVD) - this may aid drainage of CSF, thus reducing intracranial volume.
Tier 2
If tier 1 measures are failing to control the ICP, there is escalation to tier 2.
At this point, there should again be a stop to assess what the problem might be:
- Are all tier 1 measures being successfully implemented?
- Is the problem due to an intracranial or systemic issue?
- Is a repeat CT scan needed?
Measures include:
- Hyperventilation - increase minute ventilation to achieve a pCO2 of 4-4.5 kPa. The response to this needs assessing through ABGs.
- Normothermia - treat any elevated temperature with active cooling, either externally or through an intravascular device. The prevents excess cerebral metabolic activity, with theoretical benefits in blood flow.
- Osmotherapy - this is discussed in more detail in the notes below.
- Diuretics - consideration may be given to the use of furosemide if the patient is over 3 litres positive since admission. This has a theoretical benefit on oedema.
- Review CPP - a higher CPP target may be indicated in some scenarios. This will generally be a consultant decision. If there is failing autoregulation (more common for instance in diffuse axonal injury) a target CPP of 70 mmHg may be beneficial, and thus a trial could be considered. Assessment of cardiovascular status is important again at this point to ensure adequate volume status e.g. cardiac output monitoring. A higher CPP has been associated with increased rates of acute lung injury, therefore not without risk.
Tier 3
At this stage a review is again appropriate to ensure optimal care has been given so far:
- Are all tier 1 and 2 measures being delivered effectively?
- Is the CPP at an adequate value?
- Is a repeat CT scan indicated to assess for an acute lesional change?
At this stage there are limited good options, but they include:
- Decompressive craniectomy - this increases the volume of the cranium, thus reducing the pressure. The pathology is still the same and there are still significant risks.
- Barbiturate coma - Barbiturates cause profound suppression of cerebral metabolic activity, thus reducing O2 demand and blood flow.
- Therapeutic hypothermia - cooling causes a similar suppression in cerebral metabolic activity, though with a different set of complications.
Recent trials have provided challenging data on the implementation of these measures (see RescueICP and Eurotherm) which will be discussed in more detail below..
Osmotherapy
Osmotherapy can be used as an intervention to gain control of elevated ICP.
The two commonly known options are: mannitol and hypertonic saline.
The SRFT guidelines favour hypertonic saline.
This is an intervention for consultants or senior trainees.
A dose of 15ml of 30% NaCl is infused over 10 minutes.
This must be through a central venous catheter to minimise vein injury.
It is possible to repeat this (up to 4 times in 24 hours) if the serum sodium remains below 155 mmol/l and serum osmolality below 320 mosm/l.
Hypertonic saline is also available in other concentrations.
An alternative dose is 2ml/kg of 5% solution.
The effect is probably from a combination of osmotic shift of fluid (drawing fluid into the circulation from the tissue) and improved CBF.
Mannitol is also commonly used (perhaps more so).
The dose is 0.25-1 g/kg.
It is usually given as a 20% solution.
It can be repeated as boluses, taking care that the serum osmolality remains below 320 mOsm/l
The two commonly known options are: mannitol and hypertonic saline.
The SRFT guidelines favour hypertonic saline.
This is an intervention for consultants or senior trainees.
A dose of 15ml of 30% NaCl is infused over 10 minutes.
This must be through a central venous catheter to minimise vein injury.
It is possible to repeat this (up to 4 times in 24 hours) if the serum sodium remains below 155 mmol/l and serum osmolality below 320 mosm/l.
Hypertonic saline is also available in other concentrations.
An alternative dose is 2ml/kg of 5% solution.
The effect is probably from a combination of osmotic shift of fluid (drawing fluid into the circulation from the tissue) and improved CBF.
Mannitol is also commonly used (perhaps more so).
The dose is 0.25-1 g/kg.
It is usually given as a 20% solution.
It can be repeated as boluses, taking care that the serum osmolality remains below 320 mOsm/l
Decompressive Craniectomy
This has the theoretical advantage of removing the confining box that is an essential factor in causing raised ICP to develop - the brain is given space to expand.
However, 2 significant studies have cast uncertainty on the benefits of such an intervention.
The RESCUEicp study looked at decompressive craniectomy versus barbiturate coma in patients who were otherwise at maximal therapy.
Although there were the expected benefits in lowering ICP and mortality, there was an increased number of patients with poor neurological outcomes.
This therefore raised some questions about the actual benefits of the intervention, though there were notable limitations to the study.
I have reviewed the study on my blog here: http://www.rapidsequence.org.uk/blog/rescueicp
The DECRA study looked at patients undergoing a primary decompressive craniectomy (i.e. premptive) versus standard care.
This showed a worse outcome in the cohort undergoing decompression.
As such, the decision for decompression is not a straightforward one but is still an option in cases of unmanageable ICP.
However, 2 significant studies have cast uncertainty on the benefits of such an intervention.
The RESCUEicp study looked at decompressive craniectomy versus barbiturate coma in patients who were otherwise at maximal therapy.
Although there were the expected benefits in lowering ICP and mortality, there was an increased number of patients with poor neurological outcomes.
This therefore raised some questions about the actual benefits of the intervention, though there were notable limitations to the study.
I have reviewed the study on my blog here: http://www.rapidsequence.org.uk/blog/rescueicp
The DECRA study looked at patients undergoing a primary decompressive craniectomy (i.e. premptive) versus standard care.
This showed a worse outcome in the cohort undergoing decompression.
As such, the decision for decompression is not a straightforward one but is still an option in cases of unmanageable ICP.
Hypothermia
Therapeutic hypothermia also has several theoretical benefits - reduced CMR, reduced inflammation, neuroprotection.
However, the evidence doesn’t support it as a therapeutic option in TBI.
The main trial of note is the Eurotherm trial.
This suggested a worse outcome at 6 months if therapeutic hypothermia (between 32 and 25 degrees) was used as an initial tier 2 intervention.
There is some debate about this trial, particularly around the way it fitted hypothermia into current practice in relation to other measures.
As such, avoidance of hyperthermia is still often employed as a later measure in patients on a significant level of other interventions.
However, the evidence doesn’t support it as a therapeutic option in TBI.
The main trial of note is the Eurotherm trial.
This suggested a worse outcome at 6 months if therapeutic hypothermia (between 32 and 25 degrees) was used as an initial tier 2 intervention.
There is some debate about this trial, particularly around the way it fitted hypothermia into current practice in relation to other measures.
As such, avoidance of hyperthermia is still often employed as a later measure in patients on a significant level of other interventions.
Links & References
- NICE. Head injury: assessment and early management [CG176]. 2014. Available at: https://www.nice.org.uk/guidance/cg176/chapter/1-recommendations.
- Moppett, I. Traumatic brain injury: assessment, resuscitation and early management. BJA. 2007. 99(1):18-31.
- Manara, A. Thomas, M. Principles of the Management of Head Injury and Brain Protection. E-learning for healthcare. 2016
- Carney, N. et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Brain Trauma Foundation. 2016. Available at: http://www.braintrauma.org/
- Naisbett, J. Ferris, P. Traumatic Brain Injury Guidelines. 2015. Available at: http://www.neuroicu.guru/
- Flower, O. Finfer, S. Transfusion thresholds in acute brain injury. SMACC DUB. 2016. Available at: https://www.smacc.net.au/2017/03/transfusion-thresholds-acute-brain-injury/
- Dinsmore, J. Traumatic brain injury: an evidence based review of management. CEACCP. 2013. 13(6):189-195. Available at:
- Heaton, T. RESCUEicp. Rapid Sequence. 2017. Available at: http://www.rapidsequence.org.uk/blog/rescueicp
- Hutchinson, P. et al. Trial of decompressive craniectomy for traumatic intracranial hypertension (RESCUEicp Trial). 2016. NEJM. 375: 1119-1130. Available at:http://www.nejm.org/doi/full/10.1056/NEJMoa1605215#t=article
- TheBottomLine. RESCUEicp. 2016. A great appraisal of the key points of the paper. Available at: http://www.thebottomline.org.uk/summaries/icm/rescue-icp/
- Andrews, P. et al. Hypothermia for intracranial hypotension after traumatic brain injury. NEJM. 2015. 373: 2403-2312. Available at: http://www.nejm.org/doi/full/10.1056/NEJMoa1507581
- Horner, D. JC: Eurotherm or Euroburned. StEmlyn’s. 2015. Available at: http://stemlynsblog.org/jc-eurotherm-or-euroburned-st-emlyns/
- The Brain Trauma Foundation. Useful guidance on traumatic brain injury. (Available at http://www.braintrauma.org/)