Toxidromes
A toxidrome is a clinical picture resulting from a toxic trigger.
The name comes from a combination of the words toxic and syndrome.
The toxidrome can result from ingestion of drugs or indeed other clinical condition, such as drug withdrawal (alcohol withdrawal is sympathomimetic)
The toxidrome concept can be useful because of the varying effects that result when a combination of drugs is taken - the prevailing toxidrome often being the most important to manage. However, the clinical picture can still be very complicated in mixed ingestion, limiting this applicability and requiring individual clinical assessment.
The standard approach to managing a poisoned patient still needs to be applied.
There are 5 main toxidromes:
The name comes from a combination of the words toxic and syndrome.
The toxidrome can result from ingestion of drugs or indeed other clinical condition, such as drug withdrawal (alcohol withdrawal is sympathomimetic)
The toxidrome concept can be useful because of the varying effects that result when a combination of drugs is taken - the prevailing toxidrome often being the most important to manage. However, the clinical picture can still be very complicated in mixed ingestion, limiting this applicability and requiring individual clinical assessment.
The standard approach to managing a poisoned patient still needs to be applied.
There are 5 main toxidromes:
- Anticholinergic
- Cholinergic
- Narcotic
- Hypnotic
- Sympathomimetic
Anticholinergic
The toxidrome is, unsurprisingly, a consequence of excessive choline blockade at muscarinic receptors.
The effects result from this blockade in both the central and peripheral nervous systems.
A wide range of drugs cause this through competition with acetylcholine at the receptor site, and as such has little impact on the other acetylcholine receptors.
The extent of the central effects is dependent on the ability of the drug to cross the blood brain barrier.
The well known mnemonic for this toxidrome is:
Blind as a bat, mad as a hatter, red as a beet, hot as hell, and dry as a bone.
This represents mydriasis (with subsequent loss of accommodation), altered mental state, flushing, pyrexia, dry skin and mucous membranes, which are the most common manifestations.
Other common manifestations include:
There is a very wide range of drugs which can cause this toxidrome. As well as the specific anticholinergic drugs, a very wide range of drugs have anticholinergic side effects.
Anticholinergics - atropine, glycopyrolate.
Antihistamines - promethazine, chlorphenarimine,hydroxyzine
Antipsychotics - quetiapine, olanzapine, clozapine, chlorpromazine.
Cyclic antidepressants - amitriptyline, doxepin, nortriptyline
Miscellaneous - carbamazepine.
Management is initially supportive and as per management of general toxic ingestion.
The specific antidote is phytostigmine salicylate - it works by inhibiting acetylcholinesterase, allowing more endogenous acetylcholine to compete at the receptors.
Most patients don’t require it - advised for compromising tacchydysrhythmias, intractable seizures, or severe agitation.
Treatment described on Toxbase is generally purely supportive though.
The effects result from this blockade in both the central and peripheral nervous systems.
A wide range of drugs cause this through competition with acetylcholine at the receptor site, and as such has little impact on the other acetylcholine receptors.
The extent of the central effects is dependent on the ability of the drug to cross the blood brain barrier.
The well known mnemonic for this toxidrome is:
Blind as a bat, mad as a hatter, red as a beet, hot as hell, and dry as a bone.
This represents mydriasis (with subsequent loss of accommodation), altered mental state, flushing, pyrexia, dry skin and mucous membranes, which are the most common manifestations.
Other common manifestations include:
- urinary retention,
- sinus tacchycardia,
- reduced GI motility,
- tremor,
- hypertension.
There is a very wide range of drugs which can cause this toxidrome. As well as the specific anticholinergic drugs, a very wide range of drugs have anticholinergic side effects.
Anticholinergics - atropine, glycopyrolate.
Antihistamines - promethazine, chlorphenarimine,hydroxyzine
Antipsychotics - quetiapine, olanzapine, clozapine, chlorpromazine.
Cyclic antidepressants - amitriptyline, doxepin, nortriptyline
Miscellaneous - carbamazepine.
Management is initially supportive and as per management of general toxic ingestion.
The specific antidote is phytostigmine salicylate - it works by inhibiting acetylcholinesterase, allowing more endogenous acetylcholine to compete at the receptors.
Most patients don’t require it - advised for compromising tacchydysrhythmias, intractable seizures, or severe agitation.
Treatment described on Toxbase is generally purely supportive though.

Image taken from www.lifeinthefastlane.com.
#FOAMed
#FOAMed
Cholinergic
Again, unsurprisingly, this syndrome is characterised by the features of excess acetylcholine activity - primarily parasympathetic nervous system effects.
The syndrome can be surmised based on this pathophysiology - increased acetylcholine activity at the peripheral muscarinic, nicotinic and central receptors.
The acronym SLUDGE is useful for thinking about the muscarinic effects.:
Salivation
Lacrimation
Urination
Diarrhoea
Gastrointestinal distress
Emesis
Other features include:
Miosis
Bradycardia
Sweating
Bronchospasm
Hypotension
Central effects include:
Anxiety
Ataxia
Tremor
Confusion
Seizures
Coma
Nicotinic effects include:
Muscle fasciculations
Weakness
Respiratory failure - secondary to diaphragmatic weakness.
The classic causes of this are the organophosphates. These chemicals include insecticides, chemical warfare agents (e.g. sarin gas), but also some medicines (e.g. ophthalmic agents).
The mainstay of treatment is anticholinergics, primarily in the form of atropine, titrated to effect.
The syndrome can be surmised based on this pathophysiology - increased acetylcholine activity at the peripheral muscarinic, nicotinic and central receptors.
The acronym SLUDGE is useful for thinking about the muscarinic effects.:
Salivation
Lacrimation
Urination
Diarrhoea
Gastrointestinal distress
Emesis
Other features include:
Miosis
Bradycardia
Sweating
Bronchospasm
Hypotension
Central effects include:
Anxiety
Ataxia
Tremor
Confusion
Seizures
Coma
Nicotinic effects include:
Muscle fasciculations
Weakness
Respiratory failure - secondary to diaphragmatic weakness.
The classic causes of this are the organophosphates. These chemicals include insecticides, chemical warfare agents (e.g. sarin gas), but also some medicines (e.g. ophthalmic agents).
The mainstay of treatment is anticholinergics, primarily in the form of atropine, titrated to effect.
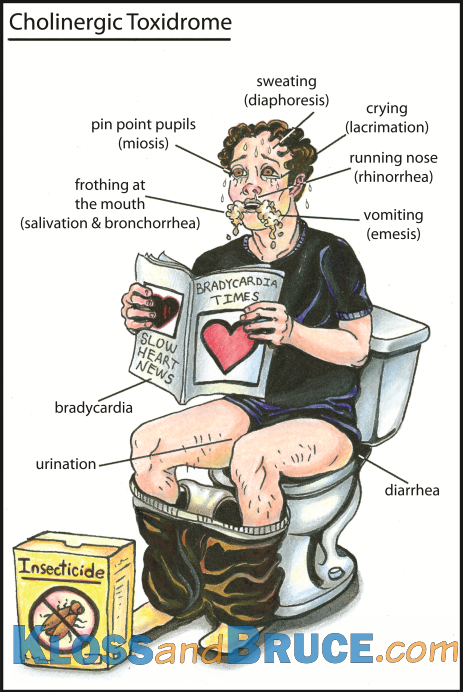
Image taken from www.lifeinthefastlane.com #FOAMed
Narcotic
The term narcotic refers to a drug that induces sleep or stupor, and generally refers to the opioid class of drugs.
Opioids are the class of drugs which have action at opioid receptors - these have the classic effects of analgesia, somnolence, respiratory depression, cardiovascular depression and reduced gastric motility.
The term opiates refers to the naturally occurring opioids - morphine, codeine and papaverine.
The classic triad for opioid toxicity is:
Other features can include:
The broader picture of the presentation will depend on other factors of the drug such as the route of administration, specific opioid and co-ingestants.
‘Aggressive’ airway control is the central component of management, as per ALS management, to provide airway protection and ventilatory support for obtunded patients.
Naloxone is the specific reversal agent - it is a competitive antagonist at the opioid receptors, blocking the effect of the ingested opioid.
It can be given by IV, IM, IO, intranasal and endotracheal routes.
The important factors for consideration when administering are:
Full rapid reversal of opioids in an opioid dependent patient is potentially hazardous because of the effects of the adverse effects of sudden withdrawal.
This can include cardiovascular instability, severe agitation, and vomiting.
In these cases, careful IV titration to effect is important
- initial dose aliquots of 100 mcg.
In non dependent users, less careful titration is needed
- can start with 400 mcg .
The IM dose is 2mg.
The clinical half life of naloxone is 20-60 mins, which can be significantly less that some of the very long acting opioids e.g. methadone.
An infusion may be needed in these cases - this is typically calculated as ⅔ of the successful reversal dose per hour.
Specific Cases
Methadone is a particularly long acting opioid and has a higher risk of cardiac arrhythmias.
Tramadol has an opioid mechanism of action as well effects mediated by reduced NA and 5HT reuptake - it has a particularly long duration and additional naloxone doses may be needed.
Modification of opioids by back-room chemists has produced some incredibly potent opioids that may require significant naloxone doses to reduce e.g. ‘china white’.
Buprenorphine is mixed agonist/antagonist which may only be partially responsive to naloxone.
Opioids are the class of drugs which have action at opioid receptors - these have the classic effects of analgesia, somnolence, respiratory depression, cardiovascular depression and reduced gastric motility.
The term opiates refers to the naturally occurring opioids - morphine, codeine and papaverine.
The classic triad for opioid toxicity is:
- CNS depression
- Respiratory depression
- Miosis
Other features can include:
- Bradycardia
- Hypotension
- Altered mental status e.g. euphoria
- Pruritis
The broader picture of the presentation will depend on other factors of the drug such as the route of administration, specific opioid and co-ingestants.
‘Aggressive’ airway control is the central component of management, as per ALS management, to provide airway protection and ventilatory support for obtunded patients.
Naloxone is the specific reversal agent - it is a competitive antagonist at the opioid receptors, blocking the effect of the ingested opioid.
It can be given by IV, IM, IO, intranasal and endotracheal routes.
The important factors for consideration when administering are:
- Baseline opioid tolerance
- Type of opioid
Full rapid reversal of opioids in an opioid dependent patient is potentially hazardous because of the effects of the adverse effects of sudden withdrawal.
This can include cardiovascular instability, severe agitation, and vomiting.
In these cases, careful IV titration to effect is important
- initial dose aliquots of 100 mcg.
In non dependent users, less careful titration is needed
- can start with 400 mcg .
The IM dose is 2mg.
The clinical half life of naloxone is 20-60 mins, which can be significantly less that some of the very long acting opioids e.g. methadone.
An infusion may be needed in these cases - this is typically calculated as ⅔ of the successful reversal dose per hour.
Specific Cases
Methadone is a particularly long acting opioid and has a higher risk of cardiac arrhythmias.
Tramadol has an opioid mechanism of action as well effects mediated by reduced NA and 5HT reuptake - it has a particularly long duration and additional naloxone doses may be needed.
Modification of opioids by back-room chemists has produced some incredibly potent opioids that may require significant naloxone doses to reduce e.g. ‘china white’.
Buprenorphine is mixed agonist/antagonist which may only be partially responsive to naloxone.
Hypnotic
The hypnotic toxidrome (also described as sedative toxidrome) is similar to but different from the narcotic one.
The most common drugs are the benzodiazepines and barbiturates, but alcohol excess can also be considered as belonging to this category as there are some similarities.
These include:
They action is through general CNS depression, most commonly by GABA receptor blockade (particularly GABA-A).
The main feature is generalised CNS depression, generally with less respiratory depression than with opioid toxicity.
Other features will be dependent on the drug(s) ingested, but can include:
Again management centres on appropriate A-E assessment and supportive treatment of any system failure e.g. intubation and ventilation.
Flumazenil is a specific ‘antidote’ for benzodiazepine toxicity, but its use is much more controversial that naloxone.
This is because its use in benzo dependent patients or in the presence of co-ingestants can precipitate withdrawal and a high risk of seizures.
If it is indicated, it is given via careful titration - 0.2mg/min.
The most common drugs are the benzodiazepines and barbiturates, but alcohol excess can also be considered as belonging to this category as there are some similarities.
These include:
- Barbiturates - Phenobarbital, Pentobarbital
- Benzos - Diazepam
- Antiepileptics
- Antihistamines
- Chloral Hydrate
They action is through general CNS depression, most commonly by GABA receptor blockade (particularly GABA-A).
The main feature is generalised CNS depression, generally with less respiratory depression than with opioid toxicity.
Other features will be dependent on the drug(s) ingested, but can include:
- Ataxia
- Confusion
- Hallucinations
- Hypotension
- Bradycardia
- Pupil changes (miosis or mydriasis)
- Hyporeflexia/flaccidity
- Respiratory depression (or even apnoea in severe overdose)
- Cardiac arrhythmias
Again management centres on appropriate A-E assessment and supportive treatment of any system failure e.g. intubation and ventilation.
Flumazenil is a specific ‘antidote’ for benzodiazepine toxicity, but its use is much more controversial that naloxone.
This is because its use in benzo dependent patients or in the presence of co-ingestants can precipitate withdrawal and a high risk of seizures.
If it is indicated, it is given via careful titration - 0.2mg/min.
Sympathomimetic
This syndrome is characterised by an excess of sympathetic nervous system activity.
There are a number of prescription and street drugs that can trigger this toxidrome in high doses:
There are several mechanisms through which these effects are mediated:
The toxidrome can be characterised by those of the sympathetic nervous system:
The usual A to E approach is the recommended starting point, treating problems that arise, and in particular looking for hypoglycaemia which can present in a similar way.
The degree of agitation can be of significant danger to the patient, requiring intervention to keep the patient safe.
Mechanical restraint is associated with an increased rate of sudden death - titrated doses of benzodiazepines are recommended.
This can often help control the other sympathomimetic features e.g. hyperthermia.
Anti-adrenergic drugs can be used, but beta-blocker use can result in unopposed alpha receptor activity.
Specific anti-hypertensive agents may be needed e.g. nitroglycerin.
There are a number of prescription and street drugs that can trigger this toxidrome in high doses:
- Cocaine
- Amphetamines
- Methamphetamines
- Ephedrine containing drugs
- Withdrawal from CNS depressant drugs e.g. alcohol
- Hypermetabolic states e.g. MH, NMS
There are several mechanisms through which these effects are mediated:
- Direct adrenoceptor agonism
- Release of norepinephrine from afferent neurons
- Prevention of noradrenaline metabolism
The toxidrome can be characterised by those of the sympathetic nervous system:
- Tachycardia
- Hypertension (and secondary features e.g. headache, ICH)
- Diaphoresis
- Agitation
- Hyperthermia
- Cardiac arrhythmias
- Seizures
- Mydriasis
The usual A to E approach is the recommended starting point, treating problems that arise, and in particular looking for hypoglycaemia which can present in a similar way.
The degree of agitation can be of significant danger to the patient, requiring intervention to keep the patient safe.
Mechanical restraint is associated with an increased rate of sudden death - titrated doses of benzodiazepines are recommended.
This can often help control the other sympathomimetic features e.g. hyperthermia.
Anti-adrenergic drugs can be used, but beta-blocker use can result in unopposed alpha receptor activity.
Specific anti-hypertensive agents may be needed e.g. nitroglycerin.
References
- Medscape - Anticholinergic toxicity. http://emedicine.medscape.com/article/812644-overview. Accessed 29/12/2015.
- Toxbase. Accessed 30/12/2015
- Lifeinthefastlane.com - Flashcards
- Medscape - Organophosphate toxicity. Accessed 3/1/2016. http://emedicine.medscape.com/article/167726-overview
- Medscape - Opioid Toxicity. Accessed 7/1/2016. http://emedicine.medscape.com/article/815784-overview
- Peck, T. Hill, S. Williams, M. Pharmacology for Anaesthesia and Intensive Care, 3rd Ed. Cambridge University Press. 2008.
- Medscape - Sedative-Hypnotic Toxicity. Accessed 8/1/2016. http://emedicine.medscape.com/article/818430-overview
- Medscape - Benzodiazepine Toxicity. Accessed 8/1/2016. http://emedicine.medscape.com/article/813255-overview
- Medscape - Toxidromes: What every critical care nurse should know. Accessed 8/1/2016. http://www.medscape.com/viewarticle/744219
- Medscape - Sympathomimetic toxicity. Accessed 8/1/2016. http://emedicine.medscape.com/article/818583-overview#a4
- Lifeinthefastlane.com - Sympathomimetic Toxidrome. Accessed 8/1/2016. http://lifeinthefastlane.com/ccc/sympathomimetic-toxidrome/
