Renal Replacement Therapy
Contents
- Indications
- Principles
- Different types
- CVVH
- Problems
Indications
With impaired renal function, such as with a significant AKI, there can be a number of complications that arise from this loss of function.
In the short term these relate to the core functions of the kidney:
In the short term these relate to the core functions of the kidney:
- Acid-base balance
- Excretion of waste products
- Electrolyte control
- Water homeostasis
Renal replacement therapy (RRT) refers to the techniques of extracorporeal blood purification that can be employed to manage these complications until native function returns (if it does).
As such the indications are classically linked to a failure of these functions despite maximal medical therapy.
A mnemonic to remember the core indications is ‘all the vowels’:
A - Acid-base disturbance
E - Electrolyte disturbance
I - Intoxication (drugs)
O - Oedema/overload
U - Uraemia
As such the indications are classically linked to a failure of these functions despite maximal medical therapy.
A mnemonic to remember the core indications is ‘all the vowels’:
A - Acid-base disturbance
E - Electrolyte disturbance
I - Intoxication (drugs)
O - Oedema/overload
U - Uraemia
However, it may be preferable to initiate therapy before such complications develop, especially given the potential severe impact that they may have on a patient who is already critically ill..
Oh’s Intensive Care Manual provides some more objective criteria for RRT:
Oh’s Intensive Care Manual provides some more objective criteria for RRT:
- Oligouria/Anuria (<200ml/12hr)
- Urea > 35 mmol/L or uraemic complications
- Creatinine > 400 micromol/L
- K+ > 6.5 mmol/L or rapidly climbing
- Na+ > 160 or < 110 mmol/L
- Pulmonary oedema
- pH < 7.1
- Temperature > 40 degrees celsius
- Overdose with dialysable toxin
Principles
There are several different methods of RRT but they share broadly similar principle - primarily being to recreate the excretion actions of the kidney.
This will occur by manipulation of the various physical factors across a semipermeable membrane.
This is most basically broke down into water removal and solute removal.
Water Removal
A driving pressure will be used to drive the water across the semipermeable membrane.
This may be a hydrostatic pressure, created by pumps, or oncotic pressure by using a high osmolality fluid as a dialysate.
Solute Removal
Again a ‘gradient’ is needed to drive movement of solute across the membrane.
This may be by creating an electro-chemical gradient across the membrane and thus having movement of solute by diffusion down this gradient - this is the case in dialysis.
Alternatively the driving force may convection, where the solute is dragged with the solvent as it is driven across the membrane by a pressure gradient - this is the case in filtration.
This will occur by manipulation of the various physical factors across a semipermeable membrane.
This is most basically broke down into water removal and solute removal.
Water Removal
A driving pressure will be used to drive the water across the semipermeable membrane.
This may be a hydrostatic pressure, created by pumps, or oncotic pressure by using a high osmolality fluid as a dialysate.
Solute Removal
Again a ‘gradient’ is needed to drive movement of solute across the membrane.
This may be by creating an electro-chemical gradient across the membrane and thus having movement of solute by diffusion down this gradient - this is the case in dialysis.
Alternatively the driving force may convection, where the solute is dragged with the solvent as it is driven across the membrane by a pressure gradient - this is the case in filtration.
Types
There is a massive range of ways in which RRT can be undertaken.
The most important initial differentiation is the mechanism of substrate removal: dialysis vs filtration.
The most important initial differentiation is the mechanism of substrate removal: dialysis vs filtration.
Dialysis
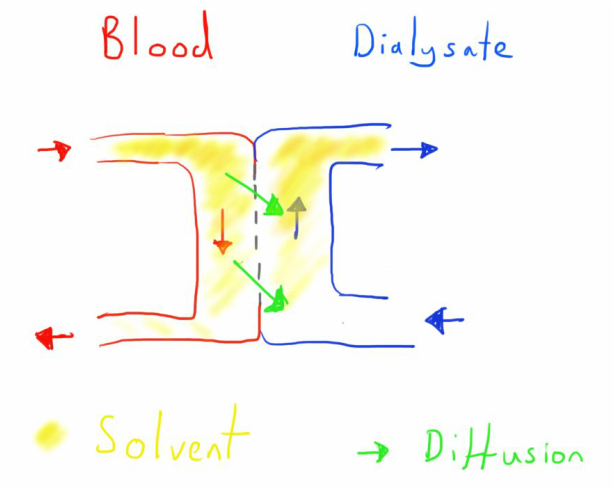
Dialysis uses the principle of diffusion to remove the solute and/or fluid.
The most effective way to do this is through a counter-current mechanism across a semipermeable membrane.
Blood is pumped into one side of the membrane, whilst dialysate is pumped into the other.
The composition of the dialysate with determine the electrochemical gradient and thus the flow of solute across the membrane.
The dialysate compartment is under negative pressure to prevent filtration of the dialysate into the blood compartment and remove excess fluid.
The dialysed blood is then returned to the patient.
This is termed haemodialysis as this process is applied to the blood (it can also be done across the peritoneum - see below)
The amount of solute removed is dependent on its molecular weight, the membrane properties, the blood flow and the dialysate flow.
Dialysis uses the principle of diffusion to remove the solute and/or fluid.
The most effective way to do this is through a counter-current mechanism across a semipermeable membrane.
Blood is pumped into one side of the membrane, whilst dialysate is pumped into the other.
The composition of the dialysate with determine the electrochemical gradient and thus the flow of solute across the membrane.
The dialysate compartment is under negative pressure to prevent filtration of the dialysate into the blood compartment and remove excess fluid.
The dialysed blood is then returned to the patient.
This is termed haemodialysis as this process is applied to the blood (it can also be done across the peritoneum - see below)
The amount of solute removed is dependent on its molecular weight, the membrane properties, the blood flow and the dialysate flow.
Filtration
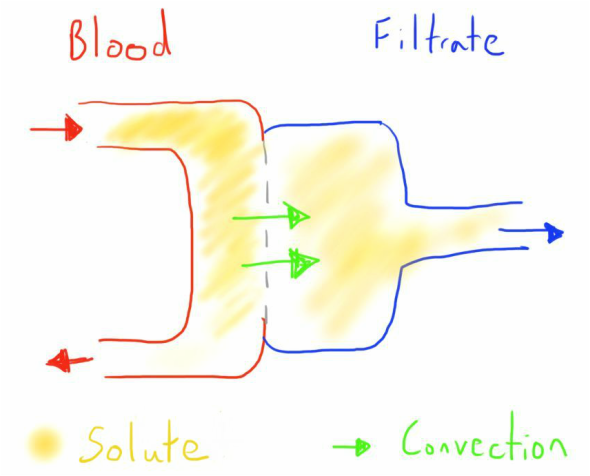
In filtration, the desired removal is achieved through a hydrostatic pressure gradient.
Again, the term haemofiltration is used as the process is applied to the blood (there isn’t an equivalent like with dialysis).
Blood is taken from the patient and a pump is used to create a high pressure within the filtration chamber.
This pressure causes movement of the water through the semipermeable membrane, dragging solutes with it by convection - this is called the ultrafiltrate.
This ultrafiltrate is then discarded and replaced by an appropriately balanced electrolyte solution.
The factors affecting the amount of fluid filtered include: blood flow, membrane surface area and pore size.
In filtration, the desired removal is achieved through a hydrostatic pressure gradient.
Again, the term haemofiltration is used as the process is applied to the blood (there isn’t an equivalent like with dialysis).
Blood is taken from the patient and a pump is used to create a high pressure within the filtration chamber.
This pressure causes movement of the water through the semipermeable membrane, dragging solutes with it by convection - this is called the ultrafiltrate.
This ultrafiltrate is then discarded and replaced by an appropriately balanced electrolyte solution.
The factors affecting the amount of fluid filtered include: blood flow, membrane surface area and pore size.
The two processes can also be combined at the same time; termed haemodiafiltration.
There are several different formats these methods can take.
The most common form is purification of the blood, referred to as haemofiltration or haemodialysis.
Blood is universally returned to a large central vein, but it can be taken from either an artery or large vein.
With arterial withdrawal of blood the arterial pressure could be used to drive the system e.g. to provide the pressure in haemofiltration.
However, in hypotensive states (common on ICU) this often led to problems with the RRT functioning properly. The large bore lines were also a high risk for vessel injury, meaning it is now rarely used.
Now blood is mainly taken from a large vein (usually at the same site as blood return) and pumps are used to create the pressure needed in the circuit.
Because of the need for long term venous access, patients with chronic kidney disease will undergo creation of an arteriovenous fistula as a point of access.
The most common form is purification of the blood, referred to as haemofiltration or haemodialysis.
Blood is universally returned to a large central vein, but it can be taken from either an artery or large vein.
With arterial withdrawal of blood the arterial pressure could be used to drive the system e.g. to provide the pressure in haemofiltration.
However, in hypotensive states (common on ICU) this often led to problems with the RRT functioning properly. The large bore lines were also a high risk for vessel injury, meaning it is now rarely used.
Now blood is mainly taken from a large vein (usually at the same site as blood return) and pumps are used to create the pressure needed in the circuit.
Because of the need for long term venous access, patients with chronic kidney disease will undergo creation of an arteriovenous fistula as a point of access.
Peritoneal dialysis is an alternative to blood purification.
This technique use the peritoneum as the semipermeable membrane. Dialysate is instilled into the peritoneal cavity and left for a period of equilibration. It is then removed, along with the relevant toxins.
It is generally much slower than haemodialysis, and not without complications, but can afford greater flexibility for patients.
This technique use the peritoneum as the semipermeable membrane. Dialysate is instilled into the peritoneal cavity and left for a period of equilibration. It is then removed, along with the relevant toxins.
It is generally much slower than haemodialysis, and not without complications, but can afford greater flexibility for patients.
Duration
Another important characteristic of RRT is its duration.
This will generally either be intermittent or continuous.
Dialysis is generally so efficient that it can be undertaken in short sessions of 3-4 hours a day or every other day.
Haemofiltration is generally less efficient so is generally a continuous technique.
However, there are more recent changes that have allowed a combination approach to optimise the benefits and drawbacks of each.
This will generally either be intermittent or continuous.
Dialysis is generally so efficient that it can be undertaken in short sessions of 3-4 hours a day or every other day.
Haemofiltration is generally less efficient so is generally a continuous technique.
However, there are more recent changes that have allowed a combination approach to optimise the benefits and drawbacks of each.
Overall this has resulted in a number of specific techniques that are used.
Intermittent Haemodialysis (IHD)
As noted, dialysis as a method is so efficient that the adequate solute and fluid removal could be achieved in just a few hours.
This allowed greater flexibility as time could be spent not attached to the RRT, and indeed it is this that is commonly used in patients with end stage CKD.
It can also allow more accurate calculation of drug dosing.
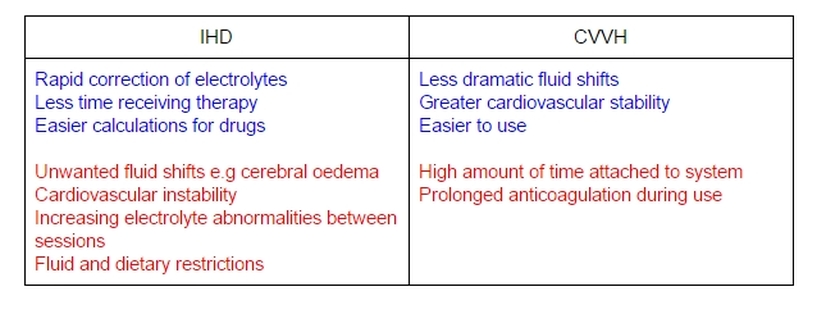
However, the rapid volume removal needed to achieve this can often result in haemodynamic instability, with episodic hypotension potentially being detrimental to renal recovery as well as other problems.
The rapid correction of solute abnormalities can also cause problems such as cerebral oedema, and may be generally poorly tolerated by critically ill patients.
Also, because the correction is episodic, there will be a continuing variation in acid-base and uraemic status, with worsening in between episodes of RRT. It also means that there has to be fluid and nutritional limitations in place for these patients.
Finally, there has been some debate that the dialysis membranes can be particularly pro-inflammatory and may cause negative effects, for example on renal recovery.
On balance, this means that IHD is generally most commonly employed for patients with stable, end stage CKD rather than critically ill patients with AKI.
This allowed greater flexibility as time could be spent not attached to the RRT, and indeed it is this that is commonly used in patients with end stage CKD.
It can also allow more accurate calculation of drug dosing.
However, the rapid volume removal needed to achieve this can often result in haemodynamic instability, with episodic hypotension potentially being detrimental to renal recovery as well as other problems.
The rapid correction of solute abnormalities can also cause problems such as cerebral oedema, and may be generally poorly tolerated by critically ill patients.
Also, because the correction is episodic, there will be a continuing variation in acid-base and uraemic status, with worsening in between episodes of RRT. It also means that there has to be fluid and nutritional limitations in place for these patients.
Finally, there has been some debate that the dialysis membranes can be particularly pro-inflammatory and may cause negative effects, for example on renal recovery.
On balance, this means that IHD is generally most commonly employed for patients with stable, end stage CKD rather than critically ill patients with AKI.
Continuous Venovenous Haemofiltration (CVVH)
This is the most common form of haemofiltration used. It’s continuous because of the relative low efficiency and venovenous because of the problems with arterial withdrawal of blood (as noted above).
The reduced efficiency does mean that it provides more gentle correction of electrolyte abnormalities with less strain on the patient.
There is also less in the way of haemodynamic instability on the patient.
The method also allows much finer control of fluid status with hourly adjustment, minimising the risk of fluid overload too.
The fluid and nutritional limitations of IHD (particularly protein rich nutrition) don’t apply.
The technique is generally less technically challenging to implement compared with IHD so can be managed by the critical care staff rather than specialist teams.
It is also actually better at removing medium to large molecular size solutes from the blood compared to dialysis.
However, the continuous nature means that patients must be attached to the circuit continuously (!) and this can be limiting for those where mobility is needed e.g. physiotherapy, transfers to scan/theatre.
This also requires continuous circuit anticoagulation, and there are a number of problems with potential bleeding risk, as well as filter circuit life because of clotting.
Generally this means that CVVH is the most common form of RRT used in critical care.
The reduced efficiency does mean that it provides more gentle correction of electrolyte abnormalities with less strain on the patient.
There is also less in the way of haemodynamic instability on the patient.
The method also allows much finer control of fluid status with hourly adjustment, minimising the risk of fluid overload too.
The fluid and nutritional limitations of IHD (particularly protein rich nutrition) don’t apply.
The technique is generally less technically challenging to implement compared with IHD so can be managed by the critical care staff rather than specialist teams.
It is also actually better at removing medium to large molecular size solutes from the blood compared to dialysis.
However, the continuous nature means that patients must be attached to the circuit continuously (!) and this can be limiting for those where mobility is needed e.g. physiotherapy, transfers to scan/theatre.
This also requires continuous circuit anticoagulation, and there are a number of problems with potential bleeding risk, as well as filter circuit life because of clotting.
Generally this means that CVVH is the most common form of RRT used in critical care.
Slow Low Efficiency Daily Dialysis (SLEDD)
Also know as slow extended daily dialysis, this is a technique increasingly used to minimise the effects of dramatic fluid and solute shifts that occur with IHD.
This is achieved by extending the duration of dialysis to between 8 and 12 hours, with reduced blood flow and reduced solute clearance.
This is achieved by extending the duration of dialysis to between 8 and 12 hours, with reduced blood flow and reduced solute clearance.
A comparison of IHD and CVVH
Dosing & Intensity
There has been much debate about the optimal dose of RRT.
Dose refers the the effective effluent/kg/h.
It was initially thought that higher dosing translated into better outcomes but this has been refuted by the 2 big RCTs on the topic (the ATN and RENAL trials).
Current practice for CRRT is to use 25-30mL/kg/h to take into account the impact of downtime on the effective removal.
Dose refers the the effective effluent/kg/h.
It was initially thought that higher dosing translated into better outcomes but this has been refuted by the 2 big RCTs on the topic (the ATN and RENAL trials).
Current practice for CRRT is to use 25-30mL/kg/h to take into account the impact of downtime on the effective removal.
CVVH
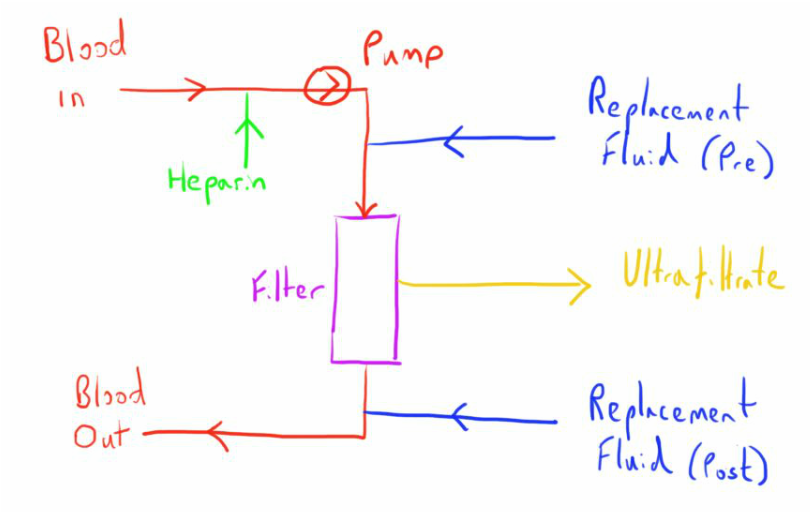
Because of it widespread use let’s look at the CVVH circuit more closely.
Some important factors about the process need further discussion.
Fluid Replacement
As can be seen for the diagram, the replacement fluid can be given either before or after filtration.
Giving the replacement fluid before filtration occurs doesn’t seem to make much sense, as it will clearly reduce the efficiency of the process (the solutes will have been diluted so less is removed by convection).
However, as the ultrafiltrate is removed in the filter the haematocrit of the blood increases and it becomes more ‘sludgy’ increasing the risk of clotting.
As such it is common to split where the fluid is replaced, with 1/3rd before the filter and 2/3rd after the filter.
This can be altered, e.g. increasing pre filter amount to try and prolong filter life.
The type of fluid used for replacement is very varied but is generally similar to plasma but without the urea.
A buffer is often added (lactate or bicarbonate) to correct the acidosis.
In most cases lactate is used, which is converted to bicarbonate by the liver.
However in certain populations e.g. liver failure, bicarbonate is used instead - this has the disadvantage of being expensive. Critical illness may also impair the liver’s ability to metabolise the lactate so levels should be watched and if levels are rising switch to bicarbonate.
They will often have potassium included because of the potassium leakage with the filters, but this will obviously want to be avoided in hyperkalaemic states.
Giving the replacement fluid before filtration occurs doesn’t seem to make much sense, as it will clearly reduce the efficiency of the process (the solutes will have been diluted so less is removed by convection).
However, as the ultrafiltrate is removed in the filter the haematocrit of the blood increases and it becomes more ‘sludgy’ increasing the risk of clotting.
As such it is common to split where the fluid is replaced, with 1/3rd before the filter and 2/3rd after the filter.
This can be altered, e.g. increasing pre filter amount to try and prolong filter life.
The type of fluid used for replacement is very varied but is generally similar to plasma but without the urea.
A buffer is often added (lactate or bicarbonate) to correct the acidosis.
In most cases lactate is used, which is converted to bicarbonate by the liver.
However in certain populations e.g. liver failure, bicarbonate is used instead - this has the disadvantage of being expensive. Critical illness may also impair the liver’s ability to metabolise the lactate so levels should be watched and if levels are rising switch to bicarbonate.
They will often have potassium included because of the potassium leakage with the filters, but this will obviously want to be avoided in hyperkalaemic states.
Anticoagulation
Both the circuit and filter are prothrombotic and blood flow can be slow and turbulent, also increasing the propensity for coagulation.
Coagulation of the filter means a reduced filter life, and replacement of the set results in loss of blood that was in the circuit and downtime.
Therefore anticoagulation is often needed to allow an acceptable circuit life (generally >24 hours).
The degree of anticoagulation is a spectrum and dependent on several factors.
Low dose anticoagulation (<500 IU/hour) is generally sufficient for most patients, with minimal impact on systemic coagulation.
Targeting an APTTr of 1.5-2 is generally preferred.
Some patients may require therapeutic anticoagulation anyway, and as such no extra is needed.
Others may be too high risk to warrant anticoagulation e.g. low platelets, coagulopathy, recent surgery.
In these cases maintaining higher flow rates of 200ml/min and good vascular access can help prolong filter life.
Indeed checking line adequacy is important in cases of repeated filter clotting, as if they are unreliable for flow, clotting will continue to occur.
Regional anticoagulation is an alternative to the systemic anticoagulation described.
This has been done previously with protamine administered in the return arm of the circuit to counteract the heparin (100 IU = 1mg).
However, citrate anticoagulation is becoming more popular.
Administering citrate to the circuit chelates the calcium ions that are vital for coagulation to occur. The calcium can then be added via a continuous infusion in the return arm of the circuit.
A specialised replacement fluid is needed for citrate anticoagulation to take into account the various electrolyte effects and differences.
Coagulation of the filter means a reduced filter life, and replacement of the set results in loss of blood that was in the circuit and downtime.
Therefore anticoagulation is often needed to allow an acceptable circuit life (generally >24 hours).
The degree of anticoagulation is a spectrum and dependent on several factors.
Low dose anticoagulation (<500 IU/hour) is generally sufficient for most patients, with minimal impact on systemic coagulation.
Targeting an APTTr of 1.5-2 is generally preferred.
Some patients may require therapeutic anticoagulation anyway, and as such no extra is needed.
Others may be too high risk to warrant anticoagulation e.g. low platelets, coagulopathy, recent surgery.
In these cases maintaining higher flow rates of 200ml/min and good vascular access can help prolong filter life.
Indeed checking line adequacy is important in cases of repeated filter clotting, as if they are unreliable for flow, clotting will continue to occur.
Regional anticoagulation is an alternative to the systemic anticoagulation described.
This has been done previously with protamine administered in the return arm of the circuit to counteract the heparin (100 IU = 1mg).
However, citrate anticoagulation is becoming more popular.
Administering citrate to the circuit chelates the calcium ions that are vital for coagulation to occur. The calcium can then be added via a continuous infusion in the return arm of the circuit.
A specialised replacement fluid is needed for citrate anticoagulation to take into account the various electrolyte effects and differences.
Fluid Removal
The filter will generally be calculated to be fluid neutral, with the replacement fluid replacing the ultrafiltrate in equal amounts.
However, it can be altered to allow fluid removal, as may be necessary in cases of pulmonary oedema or anuric failure.
This can be adapted to the patient’s fluid balance which is a big advantage of CVVH.
However, it can be altered to allow fluid removal, as may be necessary in cases of pulmonary oedema or anuric failure.
This can be adapted to the patient’s fluid balance which is a big advantage of CVVH.
Problems
Certain problems that can be encountered with CVVH include.
- Hypotension - often responds to fluid bolus. Try reducing the initial rate of fluid withdrawal until more stable. Consider alternative causes such as air embolism.
- Raised line pressure - often positional. Careful selection of site and insertion is needed. Check lines aren’t kinked.
- Raised transmembrane pressure - often a sign of filter clogging. Try increasing pre-filter replacement and increasing flow. Assess for other causes for increased clotting e.g. anticoagulation status.
- Bleeding - can result from the anticoagulation needed or platelet loss. Check coagulation screen and platelets. Always consider heparin induced thrombocytopenia if on heparin. Treat bleeding appropriately.
- Regular filter clotting - again assess for causes:
- Poor lines/kinked tubes
- Other causes of reduced blood flow
- Inadequate pre-filter fluid
- coagulopathic patient
- Rising lactate - often a sign of the liver’s inability to handle the lactate in the replacement fluid. Consider switching to bicarbonate buffer.
References
- Bersten A, Soni N. Oh’s Intensive Care Manual (7th ed).Butterworth Heinemann Elsevier. 2014
- Beers M et al. The Merck Manual (18th ed). Merck Research Laboratories. 2006
- NHS Health Education for England. e-Learning for Healthcare. Renal replacement therapies. Accessed 8th March 2016.
- Pannu N, Gibney RT. Renal replacement therapies in the intensive care unit. Therapeutics and Clinical Risk Management. 2005. 1(2): 141-150
Last updated 8th March 2016