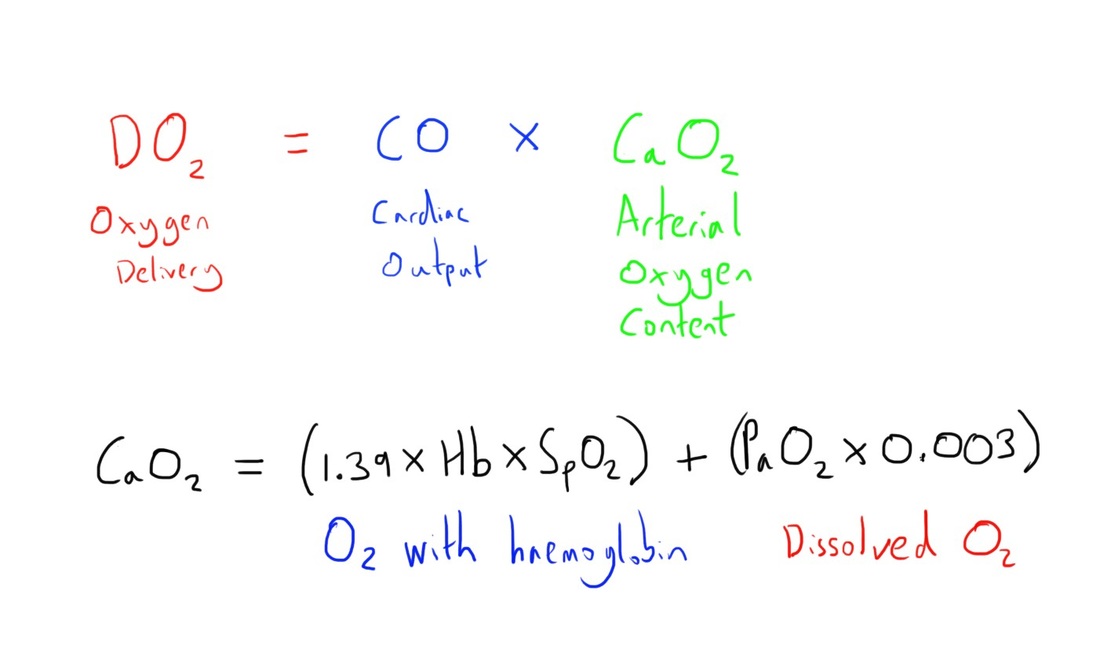Red Blood Cells
Short Notes
RBCs are one of the main components produced from donated blood.
The initial storage process involves the addition of citrate, phosphate and dextrose (CPD) to prevent coagulation from occurring.
Once the red blood cells have been centrifuged out, the RBCs are added to SAGAM (Saline, adenine, glucose and mannitol) to preserve their function.
They are then stored at 4 +/- 2oC for up to 35 days
The initial storage process involves the addition of citrate, phosphate and dextrose (CPD) to prevent coagulation from occurring.
Once the red blood cells have been centrifuged out, the RBCs are added to SAGAM (Saline, adenine, glucose and mannitol) to preserve their function.
They are then stored at 4 +/- 2oC for up to 35 days
Despite these storage measures, there is still the development of the storage lesion.
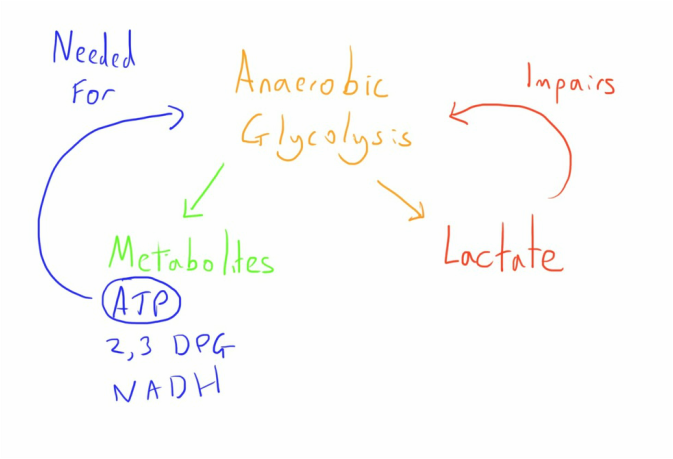
This is a result of the ongoing cellular metabolic activity which is anaerobic in nature because of the lack of mitochondria in the RBCs.
The result of this is:
This is a result of the ongoing cellular metabolic activity which is anaerobic in nature because of the lack of mitochondria in the RBCs.
The result of this is:
- An increasing lactic acidosis
- Depletion of important metabolites (ATP, 2,3-DPG, NADH)
- Impaired membrane protein function
- Increased oxidative stress
- Increased cell membrane damage.
- A high number of senescent RBCs which are rapidly neutralised after transfusion
- A high demand on the reticuloendothelial macrophage system because of this, with the potential to be overwhelmed.
- High K+ levels in the supernatant.
- Scavenging of native NO from the free haemoglobin
The decision to transfuse is based on the risks vs benefits of transfusion.
It is widely recognised that conservative transfusion thresholds are preferable to liberal ones.
As such transfusion thresholds are:
70g/l in stable patients
80g/l in patients with acute coronary syndrome.
It is widely recognised that conservative transfusion thresholds are preferable to liberal ones.
As such transfusion thresholds are:
70g/l in stable patients
80g/l in patients with acute coronary syndrome.
Long Notes
Collection
Red blood cells are one of the main components produced from donated blood.
The processes involved in blood donation and initial processing are discussed in detail elsewhere.
The processes involved in blood donation and initial processing are discussed in detail elsewhere.
Storage
After initial collection, full blood is added to citrate, phosphate and dextrose (CPD) to prevent coagulation from occurring.
Once the red blood cells have been centrifuged out, the RBCs are added to SAGAM (Saline, adenine, glucose and mannitol) to preserve their function.
They are then stored at 4 +/- 2oC for up to 35 days.
The criteria which this time frame is based on is that the amount of haemolysis must be under 1% of the total haemoglobin, and that at least 75% of the cells survive more than 24 hours after transfusion.
The volume of unit is generally between 220 and 340ml
Once the red blood cells have been centrifuged out, the RBCs are added to SAGAM (Saline, adenine, glucose and mannitol) to preserve their function.
They are then stored at 4 +/- 2oC for up to 35 days.
The criteria which this time frame is based on is that the amount of haemolysis must be under 1% of the total haemoglobin, and that at least 75% of the cells survive more than 24 hours after transfusion.
The volume of unit is generally between 220 and 340ml
The function of cell storage is to maximise the functionality and viability of the RBCs for the storage period.
However, there is progressive deterioration of these parameters over time due to:
However, there is progressive deterioration of these parameters over time due to:
- Altered metabolism
- Increased oxidative stress
- Membrane damage
1. Altered Metabolism
One problem is that RBCs lack mitochondria and therefore rely on anaerobic glycolysis to generate the important metabolites, these primarily being ATP, 2,3-DPG and NADH.
ATP is needed as the energy currency of the cell
2,3-DPG is an important molecule in the regulation of haemoglobin’s oxygen carrying capacity.
NADH is important for reversing the spontaneous oxidation of haemoglobin to methaemoglobin.
The glucose/dextrose in the storage solution provides adequate substrate for cellular metabolism but the glycolytic produces lactate as a product which accumulates. The resulting acidosis inhibits further glycolysis and so impairs production of the important metabolites.
By the end of the storage period the pH is 6.5 and the metabolites are substantially depleted.
The lack of ATP has a vicious circle effect on cellular metabolism as it is needed for the initial steps of glycolysis, leading to reduced RBC viability with increasing loss.
There is significant (often total) loss of 2,3-DPG but this is rapidly replenished on transfusion.
The loss of NADH is significant as there is increased oxidative stress as a result.
Cold storage at 4oC helps by reducing the cells metabolic rate to below one tenth of usual.
One problem is that RBCs lack mitochondria and therefore rely on anaerobic glycolysis to generate the important metabolites, these primarily being ATP, 2,3-DPG and NADH.
ATP is needed as the energy currency of the cell
2,3-DPG is an important molecule in the regulation of haemoglobin’s oxygen carrying capacity.
NADH is important for reversing the spontaneous oxidation of haemoglobin to methaemoglobin.
The glucose/dextrose in the storage solution provides adequate substrate for cellular metabolism but the glycolytic produces lactate as a product which accumulates. The resulting acidosis inhibits further glycolysis and so impairs production of the important metabolites.
By the end of the storage period the pH is 6.5 and the metabolites are substantially depleted.
The lack of ATP has a vicious circle effect on cellular metabolism as it is needed for the initial steps of glycolysis, leading to reduced RBC viability with increasing loss.
There is significant (often total) loss of 2,3-DPG but this is rapidly replenished on transfusion.
The loss of NADH is significant as there is increased oxidative stress as a result.
Cold storage at 4oC helps by reducing the cells metabolic rate to below one tenth of usual.
The cold storage does affect the function of the membrane K+/Na+ pump that maintains appropriate levels of the major cations.
The result is a gradual loss of the ions down their concentration gradients, with increased K+ outside the cell and increased Na+ inside.
The increased extracellular K+ can cause hyperkalaemia after transfusion and the increased intracellular Na+ results in swelling of the RBCs
The result is a gradual loss of the ions down their concentration gradients, with increased K+ outside the cell and increased Na+ inside.
The increased extracellular K+ can cause hyperkalaemia after transfusion and the increased intracellular Na+ results in swelling of the RBCs
2. Increased oxidative stress
For haemoglobin to function it must be in the ferrous (Fe2+) form.
However, under normal conditions a small amount oxidises to the ferric (Fe3+) form, methaemoglobin.
This cannot bind oxygen and in addition is highly unstable and, along with reactive oxygen species, ultimately producing hydroxyl radicals which are damaging to tissues.
Normally this process is slow and reversed by the NADH dependent cytochrome-b5 reductase, and a number of antioxidants neutralise the reactive oxygen species.
However, the number of these antioxidants and NADH is reduced as blood is stored for longer, impairing their ability to prevent this process.
The oxidation of haemoglobin is increased under conditions of high oxygen partial pressures and acidosis, which are present with increasing storage, thus resulting in increasing degredation of haemoglobin.
The ultimate result is increased production of reaction species which cause cell membrane damage.
For haemoglobin to function it must be in the ferrous (Fe2+) form.
However, under normal conditions a small amount oxidises to the ferric (Fe3+) form, methaemoglobin.
This cannot bind oxygen and in addition is highly unstable and, along with reactive oxygen species, ultimately producing hydroxyl radicals which are damaging to tissues.
Normally this process is slow and reversed by the NADH dependent cytochrome-b5 reductase, and a number of antioxidants neutralise the reactive oxygen species.
However, the number of these antioxidants and NADH is reduced as blood is stored for longer, impairing their ability to prevent this process.
The oxidation of haemoglobin is increased under conditions of high oxygen partial pressures and acidosis, which are present with increasing storage, thus resulting in increasing degredation of haemoglobin.
The ultimate result is increased production of reaction species which cause cell membrane damage.
3. Cell membrane damage
The stability of the membrane is vital to RBC function and viability and yet is significantly affected by the storage process through processes described below.
Increased external expression of phosphatidylserine, usually only found in the internal section of the RBC lipid membrane, increases the thrombogenicity of the RBC and also triggers removal of the cell by the body’s reticuloendothelial system.
Increased cholesterol ratios in the membrane impair RBC deformability, altering their shape.
The increased production of methaemoglobin can result in deposits of it near the cell membrane which impairs many of the important structural proteins, an important one being the transmembrane protein anion exchange 1 (AE1).
The result of this is impairment of membrane stability due to the effect on structural proteins and cell signalling that results in increased removal of the cell from the circulation (basically the cells telling the body that they are old and need getting rid of).
There is also increase production of microvesicles, small packets of cell membrane which contain large amounts of haemoglobin and phosphatidylserine.
These are responsible for some physiological disturbance after transfusion by a number of mechanisms:
The stability of the membrane is vital to RBC function and viability and yet is significantly affected by the storage process through processes described below.
Increased external expression of phosphatidylserine, usually only found in the internal section of the RBC lipid membrane, increases the thrombogenicity of the RBC and also triggers removal of the cell by the body’s reticuloendothelial system.
Increased cholesterol ratios in the membrane impair RBC deformability, altering their shape.
The increased production of methaemoglobin can result in deposits of it near the cell membrane which impairs many of the important structural proteins, an important one being the transmembrane protein anion exchange 1 (AE1).
The result of this is impairment of membrane stability due to the effect on structural proteins and cell signalling that results in increased removal of the cell from the circulation (basically the cells telling the body that they are old and need getting rid of).
There is also increase production of microvesicles, small packets of cell membrane which contain large amounts of haemoglobin and phosphatidylserine.
These are responsible for some physiological disturbance after transfusion by a number of mechanisms:
- Removal of much endogenous nitric oxide (NO) through reaction with the ‘free’ haemoglobin, leading to loss of the vasodilator effect and potentially tissue ischaemia.
- Saturation of the body’s capacity for removal of haemolysed RBCs
- Increased thrombogenicity through the expressed phosphatidylserine.
In summary, the main results of all these changes on the blood itself are:
- High levels of non-viable cells in the stored blood (up to 25% at 4 weeks)
- High levels of haemoglobin in the microvesicles in the supernatant, a potent scavenger of NO.
- Increased thrombogenicity of the blood cells through phosphatidylserine expression.
- Increased K+ in the supernatant.
Clinical Effects
As can be well appreciated, even before consideration of the many forms of immune related transfusion reactions, there can be several problems arising from the process of storing blood, described as the red cell storage lesion.
Much of this relates to the high levels of non-viable RBCs in the stored blood.
The must be removed from the circulation by the body’s regulatory haemolysis process, and this occurs within 1 hour of transfusion.
The high number of microvesicles also need removal from the circulation, and this is done by the same system, primarily the reticuloendothelial macrophage system.
This massive increase in demand on the system can lead to it being overwhelmed.
The result of this may be an increased amount of the damaging byproducts, particularly the iron based molecules such as free haemoglobin, haemin and free iron.
The impact of these includes scavenging of NO with endothelial dysfunction and platelet activation, as well as inappropriate macrophage responses, with cytokine release and possibly impaired function.
Much of this relates to the high levels of non-viable RBCs in the stored blood.
The must be removed from the circulation by the body’s regulatory haemolysis process, and this occurs within 1 hour of transfusion.
The high number of microvesicles also need removal from the circulation, and this is done by the same system, primarily the reticuloendothelial macrophage system.
This massive increase in demand on the system can lead to it being overwhelmed.
The result of this may be an increased amount of the damaging byproducts, particularly the iron based molecules such as free haemoglobin, haemin and free iron.
The impact of these includes scavenging of NO with endothelial dysfunction and platelet activation, as well as inappropriate macrophage responses, with cytokine release and possibly impaired function.
The actual clinical impact of this storage lesion has been extensively investigated without a clear answer emerging.
Many of the studies performed have been limited by methodological flaws and confounding factors, but no clear evidence of increased harm from transfusing older blood has been demonstrated.
However, there are several large RCTs on the subject yet to publish their results.
The most recent, the ABLE trial, suggested no difference in 90-day mortality from old or new blood.
Many of the studies performed have been limited by methodological flaws and confounding factors, but no clear evidence of increased harm from transfusing older blood has been demonstrated.
However, there are several large RCTs on the subject yet to publish their results.
The most recent, the ABLE trial, suggested no difference in 90-day mortality from old or new blood.
Transfusion
The basis for the role of RBC transfusion is primarily to increase the oxygen carrying capacity of the blood.
This capacity is summarised by the equation below.
This capacity is summarised by the equation below.
As can be seen, haemoglobin is responsible for the vast majority of the blood’s oxygen carrying capacity, and hence the concept of treating anaemia.
However, as can be seen, it is not the only component of the oxygen delivery equation.
Indeed, experimental studies have suggested fit and well adults can tolerate haemoglobin levels as low as 40g/L without significant complications if there was appropriate volume replacement.
This is certainly not the case in those without the ability to compensate to such a degree, such as the critically ill, older patients, or those with specific limiting comorbidities.
This is also a simplistic analogy when it comes to assessing transfusion need as allogenic RBCs have altered rheological properties, so the theoretical benefits may not be as great as predicted, and indeed may be offset by the increased blood viscosity and impaired microcirculatory flow that comes from increasing the haematocrit (not to mention the possible impact of the storage lesion).
Indeed, there is the suggestion that cellular oxygen uptake isn’t significantly affected by the RBC transfusion, as it would appear to only be in extreme situations where the oxygen uptake is dependent on oxygen delivery given the physiological reserve.
However, as can be seen, it is not the only component of the oxygen delivery equation.
Indeed, experimental studies have suggested fit and well adults can tolerate haemoglobin levels as low as 40g/L without significant complications if there was appropriate volume replacement.
This is certainly not the case in those without the ability to compensate to such a degree, such as the critically ill, older patients, or those with specific limiting comorbidities.
This is also a simplistic analogy when it comes to assessing transfusion need as allogenic RBCs have altered rheological properties, so the theoretical benefits may not be as great as predicted, and indeed may be offset by the increased blood viscosity and impaired microcirculatory flow that comes from increasing the haematocrit (not to mention the possible impact of the storage lesion).
Indeed, there is the suggestion that cellular oxygen uptake isn’t significantly affected by the RBC transfusion, as it would appear to only be in extreme situations where the oxygen uptake is dependent on oxygen delivery given the physiological reserve.
So in general there are several points for consideration with RBC transfusion:
- The theoretical benefits of increasing the haemoglobin to increase the oxygen carrying capacity of the blood may not really be achieved by allogenic RBC transfusion.
- The theoretical benefits of increasing the oxygen carrying capacity of the blood may not actually have much impact on actual oxygen delivery to the tissues except in extreme situations.
- The effect of the ‘storage lesion’ from RBC storage is unclear
- There are known harms associated with blood product transfusion, including life threatening reactions and disease transmission.
- There is fairly recurrent (though incomplete) clinical evidence that there is no benefit derived from a liberal transfusion policy i.e. transfusing at a higher Hb.
- The evidence for modification of transfusion thresholds based on underlying disease e.g. ischaemic heart disease, isn’t really present.
These points highlight the fact that the decision for transfusion should be a careful balancing of the significant risks with the desired benefits.
However, there have been efforts to provide guidance based on the risks/benefits of transfusion, and as noted these are of a strongly conservative slant.
However, there have been efforts to provide guidance based on the risks/benefits of transfusion, and as noted these are of a strongly conservative slant.
Nice Recommendations
Try implement alternatives to RBC transfusion where possible, including:
Iron therapy:
Iron therapy:
- Offer oral therapy to patients pre-operatively if they have iron deficiency anaemia
- Consider intravenous iron therapy if the oral route isn’t tolerated, if the iron deficiency is functional in nature, or if more rapid correction is needed.
Minimise Blood loss:
Offer tranexamic acid to patients undergoing surgery when >500ml blood loss is anticipated
Consider using cell salvage if a large volume of blood loss is expected
Offer tranexamic acid to patients undergoing surgery when >500ml blood loss is anticipated
Consider using cell salvage if a large volume of blood loss is expected
Thresholds
Use a Hb transfusion threshold of 70g/L for transfusion in most cases, with a target of 70-90g/l post transfusion.
Use a Hb transfusion threshold of 80g/l for transfusion in cases of acute coronary syndrome, with a target of 80-100g/l post transfusion.
Consider tailored thresholds for patients with chronic anaemia.
Such thresholds and targets aren’t appropriate for patients with massive haemorrhage.
Use a Hb transfusion threshold of 70g/L for transfusion in most cases, with a target of 70-90g/l post transfusion.
Use a Hb transfusion threshold of 80g/l for transfusion in cases of acute coronary syndrome, with a target of 80-100g/l post transfusion.
Consider tailored thresholds for patients with chronic anaemia.
Such thresholds and targets aren’t appropriate for patients with massive haemorrhage.
Author: Tom Heaton
Last Updated: 19th May 2016
Last Updated: 19th May 2016
References
- L Green et al. Modern banking, collection, compatability testing and storage of blood and blood components. Anaesthesia. 2015. 70 (suppl 1.): 3-9.
- D Orlav, K Karkouti. The pathophysiology and consequences of red blood cell storage. Anaesthesia. 2015. 70 (suppl 1.): 29-37
- A Shah et al. Evidence and triggers for the transfusion of blood and blood products. Anaesthesia. 2015. 70 (suppl 1.): 10-19
- NICE. NICE Guideline (NG24) – Blood transfusion. 2015. (Available at https://www.nice.org.uk/guidance/ng24. Accessed 18th May 2016).