Inhalational Anaesthetic Agents
Last updated - 18th December 2017 Tom Heaton
Mechanism of Action
Previous theories of action have focused on the particular relationship between their lipid solubility and potency.
This correlation was so profound that it was thought to represent a physicochemical effect of the agents, and termed the Meyer-Overton Hypothesis.
The theory was that the agents had to cross the lipid blood brain barrier to have their effect, and so their lipid solubility affected their potency.
Other theories suggested that the molecules dissolved into the phospholipid bilayer of cells, expanding them and thus providing anaesthesia through this disruption.
The current understanding of the action of general anaesthetic agents is more complex than this, and probably relates to lipophilic properties of important protein-based binding sites.
In a similar way to intravenous agents, this is through the potentiation of ‘inhibitory’ ligand gated ion channels, and inhibition of ‘excitatory’ ones.
The potentiation of the GABAa receptor and glycine receptor (both important inhibitory CNS neurotransmitter receptors) is recognised as being a mechanism of action of the halogenated volatile anaesthetic agents.
This is particularly the case with glycine, and it has been described that the volatile agents may have more of an action in the spinal cord, unlike with IV anaesthetic agents.
This correlation was so profound that it was thought to represent a physicochemical effect of the agents, and termed the Meyer-Overton Hypothesis.
The theory was that the agents had to cross the lipid blood brain barrier to have their effect, and so their lipid solubility affected their potency.
Other theories suggested that the molecules dissolved into the phospholipid bilayer of cells, expanding them and thus providing anaesthesia through this disruption.
The current understanding of the action of general anaesthetic agents is more complex than this, and probably relates to lipophilic properties of important protein-based binding sites.
In a similar way to intravenous agents, this is through the potentiation of ‘inhibitory’ ligand gated ion channels, and inhibition of ‘excitatory’ ones.
The potentiation of the GABAa receptor and glycine receptor (both important inhibitory CNS neurotransmitter receptors) is recognised as being a mechanism of action of the halogenated volatile anaesthetic agents.
This is particularly the case with glycine, and it has been described that the volatile agents may have more of an action in the spinal cord, unlike with IV anaesthetic agents.
Minimum Alveolar Concentration (MAC)
This is defined as - “the minimum alveolar concentration of an agent, at a steady state, that prevents reaction to a standard surgical stimulus (forearm skin incision) in 50% of subjects, at one atmosphere.”
This is a measure of anaesthetic potency.
Though in the definition it is described as the concentration, it is the partial pressure of the agent that is important.
There are several factors which affect MAC.
Increase MAC:
Reduce MAC:
This is a measure of anaesthetic potency.
Though in the definition it is described as the concentration, it is the partial pressure of the agent that is important.
There are several factors which affect MAC.
Increase MAC:
- Infancy
- Hyperthermia
- Hyperthyroidism
- Chronic opioid use
- Chronic alcohol use
- Acute amphetamine use
- Hypernatraemia
- Catecholamines
- Hypercapnia
Reduce MAC:
- Increasing age
- Neonatal period
- Pregnancy
- Hypotension
- Hypothyroidism
- Hypothermia
- Alpha -2 agonists
- Acute opioid use
- Concomitant sedatives
- Lithium
- Hypoxia
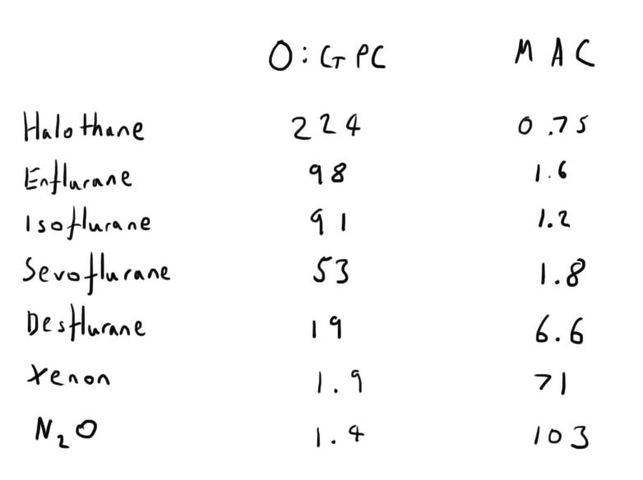
Oil:Gas Partition Coefficient (O:GPC)
The MAC is the clinical concept of potency, but the potency of volatile anaesthetic agents has long been recognised as being related to their lipid solubility.
A partition coefficient is a dimensionless quality.
It describes the solubility of a substance in two different material (i.e. the ratio of how they will dissolve in the two substances.
These substances must be:
A higher oil:gas partition coefficient represents an increased solubility in oil (i.e. an increased lipid solubility).
This represents increased anaesthetic potency, and therefore a lower MAC.
A partition coefficient is a dimensionless quality.
It describes the solubility of a substance in two different material (i.e. the ratio of how they will dissolve in the two substances.
These substances must be:
- Of equal volume
- At equilibrium
- At a defined temperature
A higher oil:gas partition coefficient represents an increased solubility in oil (i.e. an increased lipid solubility).
This represents increased anaesthetic potency, and therefore a lower MAC.
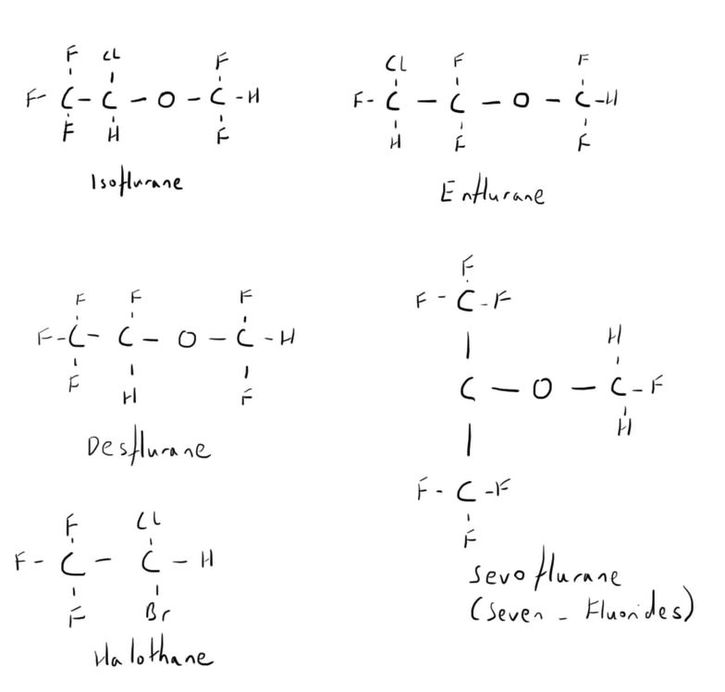
Chemistry
Nitrous oxide and xenon are exceptions to the majority of inhaled anaesthetic agents and are discussed separately.
All the others are highly halogenated molecules.
All except halothane are ethers.
Halothane is a simple hydrocarbon.
All the others are highly halogenated molecules.
All except halothane are ethers.
Halothane is a simple hydrocarbon.
Of note in the chemistry:
Ethers are larger molecules, and thus less lipid soluble. This explains why halothane is the most potent.
However, their larger size also makes them less water soluble (as less likely to polarise), and thus have a faster onset and offset.
Ethers are larger molecules, and thus less lipid soluble. This explains why halothane is the most potent.
However, their larger size also makes them less water soluble (as less likely to polarise), and thus have a faster onset and offset.
Metabolism
The vast majority of excretion is via the lungs.
However, there is a varying degree of metabolism of the agents within the body by the liver.
The is by one of the cytochrome p450 enzymes - CYP2E1.
This attacks the carbon-halogen bond.
The product is halogen ions.
It is responsible for the production trifluoroacetic acid.
The carbon-fluoride bond is the most stable and has minimal metabolism.
However, there is a varying degree of metabolism of the agents within the body by the liver.
The is by one of the cytochrome p450 enzymes - CYP2E1.
This attacks the carbon-halogen bond.
The product is halogen ions.
It is responsible for the production trifluoroacetic acid.
The carbon-fluoride bond is the most stable and has minimal metabolism.
Pharmacokinetics
This primarily relates to the relationship between the inhalation of the agent and the brain concentration.
The partial pressure of the agent in the brain (Pb) is what will impact on anaesthesia.
This will be dependent on the partial pressure of the agent in the blood (Pa), which will be dependent on the partial pressure in the alveoli (PA).
When these factors are at equilibrium and at a steady state, the partial pressures will be nearly identical.
However, at the start of anaesthesia, there will be a concentration gradient down from the alveoli to the blood, to the brain.
This gradient will be reversed at the end of anaesthesia, producing a washout curve.
Equilibrium will eventually be achieved after a period of time, in a negative exponential manner.
The speed that this equilibrium will be achieved is dependent on several factors:
The partial pressure of the agent in the brain (Pb) is what will impact on anaesthesia.
This will be dependent on the partial pressure of the agent in the blood (Pa), which will be dependent on the partial pressure in the alveoli (PA).
When these factors are at equilibrium and at a steady state, the partial pressures will be nearly identical.
However, at the start of anaesthesia, there will be a concentration gradient down from the alveoli to the blood, to the brain.
This gradient will be reversed at the end of anaesthesia, producing a washout curve.
Equilibrium will eventually be achieved after a period of time, in a negative exponential manner.
The speed that this equilibrium will be achieved is dependent on several factors:
- Equipment and agent delivery
- Patient’s physiology
- Agent properties
Equipment & Delivery
The higher the concentration of agent delivered from the vaporiser, the faster the rise in Pb because of the increased concentration gradient which it will move down.
This is the concept of overpressure, and is used at the start of anaesthesia to more rapidly elevate the Pb of an agent.
The anaesthetic agent will also be ‘diluted’ by the gas that is already within the anaesthetic circuit.
As such, the fresh gas flow to the circuit is also important.
The agent may also be ‘taken up’ by materials within the circuit (rubber, plastic, CO2 absorbers) and thus reducing the level reaching the alveoli.
This is the concept of overpressure, and is used at the start of anaesthesia to more rapidly elevate the Pb of an agent.
The anaesthetic agent will also be ‘diluted’ by the gas that is already within the anaesthetic circuit.
As such, the fresh gas flow to the circuit is also important.
The agent may also be ‘taken up’ by materials within the circuit (rubber, plastic, CO2 absorbers) and thus reducing the level reaching the alveoli.
Patient's Physiology
Several patient factors will impact on the speed of equilibrium.
- Minute ventilation
- A higher MV results in faster equilibration because of the increased amount of agent delivered
- A higher MV results in faster equilibration because of the increased amount of agent delivered
- Cardiac output
- A higher cardiac output more quickly removes the agent, thus taking longer for the concentration to build up and prolonging the time to equilibrium.
- However, as newer anaesthetic agents are less water soluble, this is less relevant.
- A higher cardiac output more quickly removes the agent, thus taking longer for the concentration to build up and prolonging the time to equilibrium.
- Functional residual capacity
- The FRC will ‘dilute’ the anaesthetic agent in the inhaled tidal volume, as so will slow the rate of equilibration.
- The FRC will ‘dilute’ the anaesthetic agent in the inhaled tidal volume, as so will slow the rate of equilibration.
- Cerebral blood flow
- A higher CBF will deliver more agent to the brain and so speed up equilibration.
- It is for this reason that pCO2 may impact upon the speed of equilibrium
- A higher CBF will deliver more agent to the brain and so speed up equilibration.
Agent Properties
It is the blood:gas partition coefficient that is the most important factor.
This is essentially the water solubility of the agent.
The more insoluble the agent is, the less will dissolve in the blood and so the higher the partial pressure will be.
As such, agents with a low blood:gas partition coefficient will have a faster onset and offset.
From slowest to fastest (with their B:GPC) are:
Note: though N2O has a higher B:GPC than desflurane, it has a faster onset and offset because of the concentration effect.
This is essentially the water solubility of the agent.
The more insoluble the agent is, the less will dissolve in the blood and so the higher the partial pressure will be.
As such, agents with a low blood:gas partition coefficient will have a faster onset and offset.
From slowest to fastest (with their B:GPC) are:
- Halothane - 2.4
- Enflurane - 1.9
- Isoflurane - 1.4
- Sevoflurane - 0.69
- Desflurane - 0.42
- N2O - 0.47
Note: though N2O has a higher B:GPC than desflurane, it has a faster onset and offset because of the concentration effect.
The Ideal Anaesthetic Agent
This understanding of how the inhalational anaesthetic agents work can let us compare the properties of the different agents.
The ideal agent will have/be:
The ideal agent will have/be:
- A high O:GPC - low MAC
- A low B:GPC - rapid onset and offset
- Analgesic properties
- Not metabolised
- Unreactive (with circuit components)
- No non-neurological effects
- Pleasant odour
- Cheap
- Stable in heat and light
- Inflammable
Links & References
- Peck, T. Hill, S. Williams, M. Pharmacology for anaesthesia and intensive care (3rd ed). 2008. Cambridge University Press.
- E-learning for healthcare. e-lfh.org.uk