Haemoglobin
Last updated 21st August 2021 - Tom Heaton
Haemoglobin (Hb) is an important protein with a number of key functions.
This video from KhanAcademy provides a nice simple introduction to Hb.
This video from KhanAcademy provides a nice simple introduction to Hb.
Structure
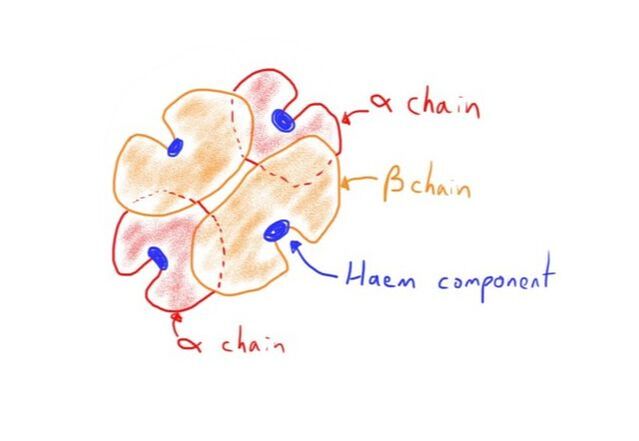
Hb has a complex quaternary structure.
A single Hb molecule comprises 4 haemoglobin chains.
Each haemoglobin chain consists of:
A single Hb molecule comprises 4 haemoglobin chains.
Each haemoglobin chain consists of:
- A haem group
- A globin chain
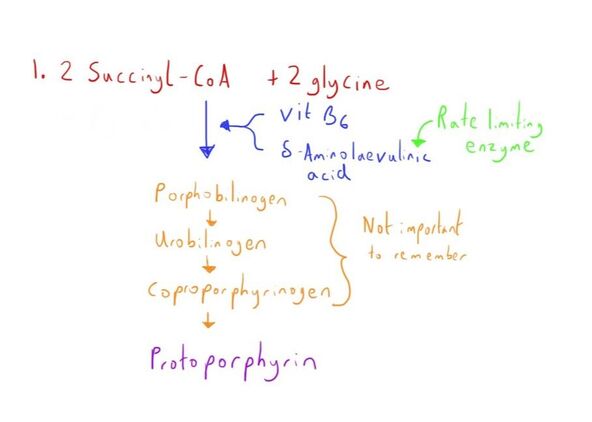
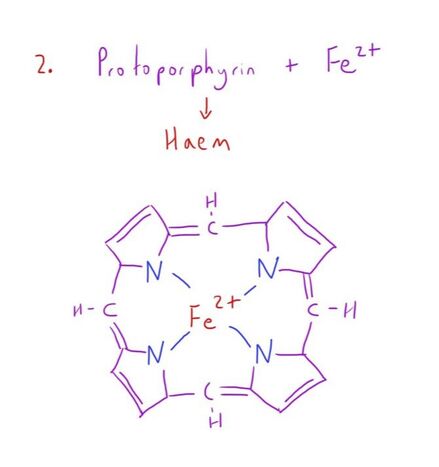
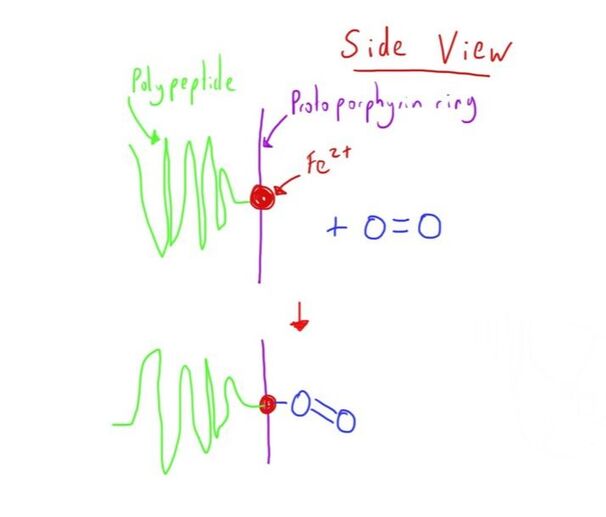
The haem group is a protoporphyrin ring with an iron (Fe2+) ion in its ferrous state at its centre.
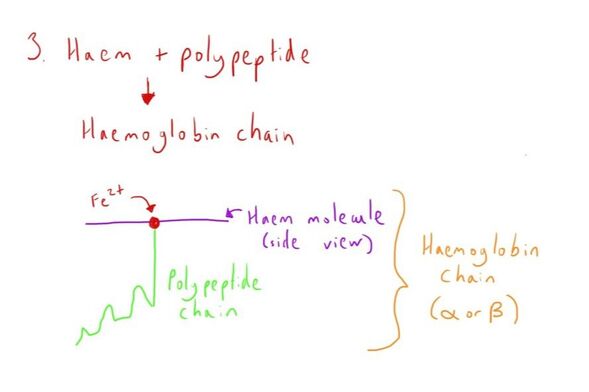
The globin chain is a long polypeptide chain, which attaches to the haem group through bonding with the Fe2+.
This structure can be seen in the diagram below (on synthesis).
The Fe2+ has the ability to form 6 bonds.
5 of these are strong bonds (4 to the protoporphyrin, 1 to the globin chain) with the 6th available for an oxygen molecule.
The globin chain is a long polypeptide chain, which attaches to the haem group through bonding with the Fe2+.
This structure can be seen in the diagram below (on synthesis).
The Fe2+ has the ability to form 6 bonds.
5 of these are strong bonds (4 to the protoporphyrin, 1 to the globin chain) with the 6th available for an oxygen molecule.
There are different forms of Hb in normal physiology:
Haemoglobin A (HbA)
Makes up around 95% of adult haemoglobin.
Consists of 2 alpha and 2 beta chains.
Haemoglobin A2 (HbA2)
Makes up around 2-3.5% of adult haemoglobin.
Consist of 2 alpha and 2 delta chains.
Foetal haemoglobin (HbF)
In an adult makes up under 1% of the circulating Hb.
However, in the foetus it makes up the majority of the Hb, comprising 50-95%.
This declines steadily over the first 6 months of life.
This form of Hb has a higher affinity for oxygen than HbA, and thus is better suited to oxygen delivery in the hypoxic womb environment.
Haemoglobin A (HbA)
Makes up around 95% of adult haemoglobin.
Consists of 2 alpha and 2 beta chains.
Haemoglobin A2 (HbA2)
Makes up around 2-3.5% of adult haemoglobin.
Consist of 2 alpha and 2 delta chains.
Foetal haemoglobin (HbF)
In an adult makes up under 1% of the circulating Hb.
However, in the foetus it makes up the majority of the Hb, comprising 50-95%.
This declines steadily over the first 6 months of life.
This form of Hb has a higher affinity for oxygen than HbA, and thus is better suited to oxygen delivery in the hypoxic womb environment.
Synthesis
Synthesis of haemoglobin occurs in the erythrocytes in the bone marrow, primarily in the long bones.
The synthesis of both the haem and polypeptide chains occurs in the cytosol of these erythrocytes.
The control of erythropoiesis is primarily through erythropoietin.
This is produced by the kidneys.
The synthesis of both the haem and polypeptide chains occurs in the cytosol of these erythrocytes.
The control of erythropoiesis is primarily through erythropoietin.
This is produced by the kidneys.
Destruction
RBCs at the end of their lifespan are removed from the circulation by the reticuloendothelial system.
Because of the challenges with iron absorption the iron is heavily recycled in this process.
Protoporphyrin is broken down into biliverdin, and then reduced to bilirubin.
This is transported to the liver by albumin where it is conjugated with glucuronic acid and excreted in the bile.
In the GI tract some bilirubin is converted to stercobilin, of which some is reabsorbed and excreted in the liver as urobilinogen.
Because of the challenges with iron absorption the iron is heavily recycled in this process.
Protoporphyrin is broken down into biliverdin, and then reduced to bilirubin.
This is transported to the liver by albumin where it is conjugated with glucuronic acid and excreted in the bile.
In the GI tract some bilirubin is converted to stercobilin, of which some is reabsorbed and excreted in the liver as urobilinogen.
Links & References
- Thomas, C. Lumb, A. Physiology of haemoglobin. CEACCP. 2012. 12(5): 251-256. https://academic.oup.com/bjaed/article/12/5/251/289041
- Guyton. Hall. Textbook of medical physiology (11th ed). 2005. Elsevier
- Khan, S. Hemoglobin. KhanAcademy. 2010. Youtube. https://www.youtube.com/watch?v=LWtXthfG9_M
- Khan, S. Hemoglobin moves O2 and CO2. KhanAcademy. 2012. Youtube. https://www.youtube.com/watch?v=QP8ImP6NCk8