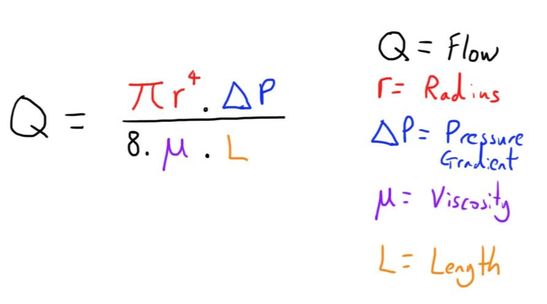Cardiopulmonary Bypass
Cardiopulmonary bypass (CPB) refers to the replacement of the function of the heart and lungs by a machine.
This allows the surgeon operating on the heart to work in much improved conditions i.e. a motionless heart without blood.
This is essential for many types of surgery.
The bypass machines are run by perfusionists who will closely control many different aspects of it.
This allows the surgeon operating on the heart to work in much improved conditions i.e. a motionless heart without blood.
This is essential for many types of surgery.
The bypass machines are run by perfusionists who will closely control many different aspects of it.
The contents of this topic include:
- The CPB Machine
- Structure
- Gas exchange
- Blood flow
- Structure
- Cardioplegia
- Non-cardioplegic myocardial protection
- Anticoagulation
- The process of CPB
The CPB Machine
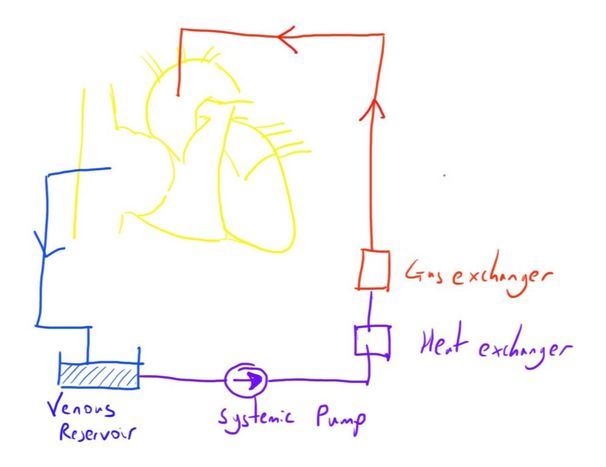
The machine involves drawing blood from a cannula in the right atrium (or other variations of the right sided circulation), and returning blood to a cannula in the ascending aorta.
Between this time, the blood has CO2 removed and O2 added, to replicate the lungs’ function, and is returned at an appropriate flow and pressure, to replicate the heart’s function.
Although this is the most basic overview, it is understandably a little more complex than this.
Some important aspects will be considered now.
Between this time, the blood has CO2 removed and O2 added, to replicate the lungs’ function, and is returned at an appropriate flow and pressure, to replicate the heart’s function.
Although this is the most basic overview, it is understandably a little more complex than this.
Some important aspects will be considered now.
Here is a very simple video introducing the CPB concept:
https://www.youtube.com/watch?v=_dMZeUETAZ0
https://www.youtube.com/watch?v=_dMZeUETAZ0
Gas Exchange
One of the primary functions of CPB is to take over the main function of the lungs; gas exchange.
This involves oxygenation of the blood, and removal of CO2.
The majority of modern CPB machines achieve this through membrane oxygenators.
This is similar to the way the lungs achieve gas exchange in some ways, by allowing diffusion of the gases down a diffusion gradient, using a large surface area to achieve effective volumes.
There are still a few different variations of these, including different structures:
And materials:
Also, of interest, historical methods includes:
The hollow fibre oxygenator is perhaps the most common type, and thus will be discussed in more detail.
This involves oxygenation of the blood, and removal of CO2.
The majority of modern CPB machines achieve this through membrane oxygenators.
This is similar to the way the lungs achieve gas exchange in some ways, by allowing diffusion of the gases down a diffusion gradient, using a large surface area to achieve effective volumes.
There are still a few different variations of these, including different structures:
- Sheet membrane oxygenators - exist as a large surface area sheet
- Hollow fibre oxygenators - the blood is passed through a large number of hollow fibres, thus producing a large surface area (similar to in renal replacement therapy).
And materials:
- Microporous membranes (e.g. polypropylene) - have small pores in them. Initially there is a direct blood gas interface, but a protein layer quickly develops.
- Silicone - act as a true membrane and thus stop plasma leak
Also, of interest, historical methods includes:
- Bubble oxygenators - oxygen is bubbled through the blood to oxygenate it
- Film oxygenation - a thin film of blood is exposed to an oxygen rich environment
The hollow fibre oxygenator is perhaps the most common type, and thus will be discussed in more detail.
As noted, many of the physical principles at play in the normal lungs are also an important factor here.
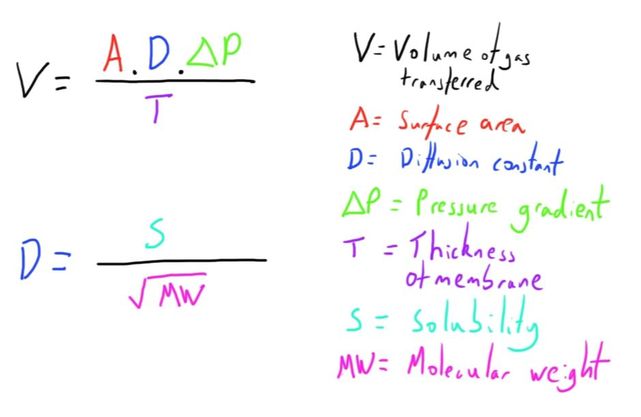
The most important consideration is that of Fick’s Law of diffusion:
The most important consideration is that of Fick’s Law of diffusion:
Healthy lungs have fantastic diffusion properties because of the very large surface area (70-100 m^2) and very small diffusion distance (10 micrometers).
Both of these are much less optimal in the hollow fibre oxygenator, with a surface area of 1.7-3.5 m^2, and a diffusion distance of 200 micrometers.
The solubility and the pressure gradient are the only remaining components in the equation.
The solubility of the gases in the membrane material is a fixed factor, and is clearly part of the design of the CPB machine.
As in the normal lungs, the solubility of CO2 is much greater than of O2.
As such, the only other modifiable factor of Fick’s Law, the pressure gradient, has to be optimised and adjusted by the perfusionist to adjust the arterial levels of these gases.
An oxygen gradient can be adjusted by altering the pO2 of the gas.
The CO2 gradient is harder to manage, but increasing the gas flow through the exchanger optimises removal of CO2 and thus maintains a greater gradient.
Both of these are much less optimal in the hollow fibre oxygenator, with a surface area of 1.7-3.5 m^2, and a diffusion distance of 200 micrometers.
The solubility and the pressure gradient are the only remaining components in the equation.
The solubility of the gases in the membrane material is a fixed factor, and is clearly part of the design of the CPB machine.
As in the normal lungs, the solubility of CO2 is much greater than of O2.
As such, the only other modifiable factor of Fick’s Law, the pressure gradient, has to be optimised and adjusted by the perfusionist to adjust the arterial levels of these gases.
An oxygen gradient can be adjusted by altering the pO2 of the gas.
The CO2 gradient is harder to manage, but increasing the gas flow through the exchanger optimises removal of CO2 and thus maintains a greater gradient.
Blood Flow
The other primary function of the CPB is to take over the pumping function of the heart.
Again, it is important to consider the physics that are at play here.
The most important equation is the Hagen-Poiseuille equation:
Again, it is important to consider the physics that are at play here.
The most important equation is the Hagen-Poiseuille equation:
The blood is drained from the right atrium under the influence of gravity.
Here it enters a venous blood reservoir and is filtered of air and debris.
After this it enters a centrifugal pump which provides the energy for the blood to be returned into the aorta.
A centrifugal pump consists of a housing with a vaned impeller inside.
This impeller is spun at great speed by an external magnet.
This confers centrifugal force to the blood which provides the kinetic energy for flow through the CPB circuit and into the aorta.
As this pump is non-occlusive, the flow it produces is more sensitive to changes in afterload (a large increase, for instance, may notably reduce flow).
There are also roller pumps with the circuit.
These may be used for driving the fluid for cardioplegia, blood from the cardiotomy reservoir, or venting of the heart.
These work by the rotatory compression of a plastic tube by a roller.
The compression and release creates a vacuum which draws blood through it, creating flow in the direction of the roller.
The degree of compression and speed of rotation can be adjusted.
This pump can deliver more constant flow rates despite changes in afterload, as it is actively displacing blood.
Here it enters a venous blood reservoir and is filtered of air and debris.
After this it enters a centrifugal pump which provides the energy for the blood to be returned into the aorta.
A centrifugal pump consists of a housing with a vaned impeller inside.
This impeller is spun at great speed by an external magnet.
This confers centrifugal force to the blood which provides the kinetic energy for flow through the CPB circuit and into the aorta.
As this pump is non-occlusive, the flow it produces is more sensitive to changes in afterload (a large increase, for instance, may notably reduce flow).
There are also roller pumps with the circuit.
These may be used for driving the fluid for cardioplegia, blood from the cardiotomy reservoir, or venting of the heart.
These work by the rotatory compression of a plastic tube by a roller.
The compression and release creates a vacuum which draws blood through it, creating flow in the direction of the roller.
The degree of compression and speed of rotation can be adjusted.
This pump can deliver more constant flow rates despite changes in afterload, as it is actively displacing blood.
Cardioplegia
Cardioplegia refers to the paralysis of the heart muscle through the administration of ‘toxic’ solution.
Is is the main form of myocardial protection used in cardiac surgery.
In cardiac surgery, blood flow to the myocardium often has to be interrupted.
If the myocardium continued its usual, very high, metabolic activity, then it would very quickly become substrate deplete and start to die.
As such, the myocardium is ‘paralysed’, whilst the CPB machine takes on the work of circulating the blood.
When combined with hypothermia, as it often is, the metabolic demands of the myocardium drop significantly, and thus allow relatively prolonged periods without blood flow to it.
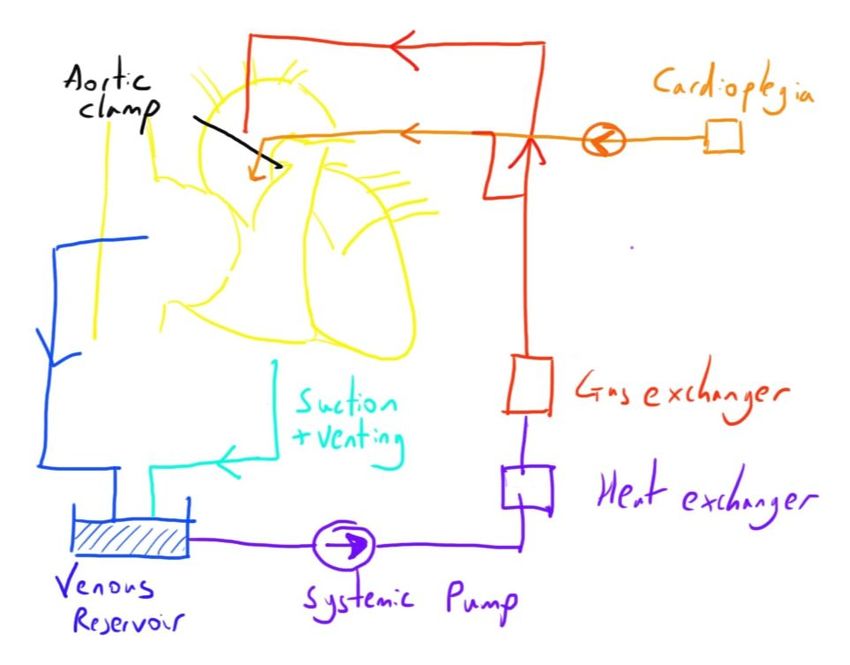
Cardioplegia solution is instilled directly into the coronary circulation via a number of ways:
This allows the solution to specifically reach the myocardial cells, and not other tissues.
Generally, the antegrade route is used.
Retrograde cardioplegia may be used if there is concern over the ability for the solution to reach the myocardium via the normal coronary circulation e.g. severe coronary artery disease or notable ventricular hypertrophy.
The cardioplegia solution typically involves a high concentration of potassium chloride, usually in 20 mmol/L concentration.
This causes a reduction in the resting membrane potential of the myocardium and loss of excitability.
It is usually administered intermittently every 15-30 minutes in order to maintain cardioplegia whilst also allowing good operating conditions.
There are several variations for the method of administering cardioplegia solution:
Crystalloid simply acts as a vehicle for the cardioplegia agent.
Blood is thought to have theoretical advantages as it can simultaneously provide substrate delivery to the myocardium e.g. oxygen, and many of the other functions of blood e.g. buffering, free radical scavenging.
The use of cold solution is part of the hypothermia applied to reduce general metabolic activity.
However, the use of cold blood delivery is thought to be less useful because of the metabolic changes that the lower temperature has e.g. left shift of the oxygen dissociation curve.
Once the aortic cross clamp is removed, blood flow from the CPB machine can drive blood through the coronaries, washing out the cardioplegia solution and allowing recovery of the myocardium’s electrical activity.
Is is the main form of myocardial protection used in cardiac surgery.
In cardiac surgery, blood flow to the myocardium often has to be interrupted.
If the myocardium continued its usual, very high, metabolic activity, then it would very quickly become substrate deplete and start to die.
As such, the myocardium is ‘paralysed’, whilst the CPB machine takes on the work of circulating the blood.
When combined with hypothermia, as it often is, the metabolic demands of the myocardium drop significantly, and thus allow relatively prolonged periods without blood flow to it.
Cardioplegia solution is instilled directly into the coronary circulation via a number of ways:
- Into the aortic root (below an aortic cross clamp) - antegrade
- Directly into the coronary ostia - antegrade
- Backwards through the coronary sinus - retrograde
This allows the solution to specifically reach the myocardial cells, and not other tissues.
Generally, the antegrade route is used.
Retrograde cardioplegia may be used if there is concern over the ability for the solution to reach the myocardium via the normal coronary circulation e.g. severe coronary artery disease or notable ventricular hypertrophy.
The cardioplegia solution typically involves a high concentration of potassium chloride, usually in 20 mmol/L concentration.
This causes a reduction in the resting membrane potential of the myocardium and loss of excitability.
It is usually administered intermittently every 15-30 minutes in order to maintain cardioplegia whilst also allowing good operating conditions.
There are several variations for the method of administering cardioplegia solution:
- Cold crystalloid
- Cold blood
- Warm blood
Crystalloid simply acts as a vehicle for the cardioplegia agent.
Blood is thought to have theoretical advantages as it can simultaneously provide substrate delivery to the myocardium e.g. oxygen, and many of the other functions of blood e.g. buffering, free radical scavenging.
The use of cold solution is part of the hypothermia applied to reduce general metabolic activity.
However, the use of cold blood delivery is thought to be less useful because of the metabolic changes that the lower temperature has e.g. left shift of the oxygen dissociation curve.
Once the aortic cross clamp is removed, blood flow from the CPB machine can drive blood through the coronaries, washing out the cardioplegia solution and allowing recovery of the myocardium’s electrical activity.
Non-cardioplegia Myocardial Protection
Cardioplegia is not the only method of myocardial protection available.
Intermittent cross-clamping is another option.
This employs moderate hypothermia (30-32 degrees celsius) to reduce metabolic activity.
A cross clamp is then applied to the aorta in the same way.
The heart is then induced into ventricular fibrillation by a fibrillator.
The fibrillating heart is much stiller than a normal beating one and there is no coronary blood flow, thus allowing conditions suitable for operating.
However, the myocardial activity is clearly much higher than that with cardioplegia and it is only feasible for a maximum time of 20 minutes before the ischaemia becomes significant.
CABG is the only surgical procedure that may involve this technique.
Intermittent cross-clamping is another option.
This employs moderate hypothermia (30-32 degrees celsius) to reduce metabolic activity.
A cross clamp is then applied to the aorta in the same way.
The heart is then induced into ventricular fibrillation by a fibrillator.
The fibrillating heart is much stiller than a normal beating one and there is no coronary blood flow, thus allowing conditions suitable for operating.
However, the myocardial activity is clearly much higher than that with cardioplegia and it is only feasible for a maximum time of 20 minutes before the ischaemia becomes significant.
CABG is the only surgical procedure that may involve this technique.
Anticoagulation
The physiology of haemostasis can be reviewed here: http://www.medicalphysiology.co.uk/haemostasis.html
In CPB, the blood is exposed to a number of prothrombotic and proinflammatory (which is in itself prothrombotic) triggers.
This includes:
These all act to initiate the haemostatic pathways, through platelet action and the enzymes of the coagulation pathway.
This leads the the development of fibrin strands, with consumption of platelets, clotting factors and fibrinogen.
As such, anticoagulation is an essential component of CPB.
There are also a number of adverse effects on the haemostatic system as a consequence of of CPB which are discussed later.
Anticoagulation is usually achieved through the administration of unfractionated heparin.
This must be done prior to initiation of CPB.
The target is an activated clotting time (ACT) of 4 times the normal range (normal is around 120s, so a target of 480s is generally used).
A dose of 300-400 units/kg is generally used as a guide for the loading dose needed for anticoagulation to this level.
However, there is a degree of variability in response between patients.
Point of care tests which more accurately measure the dose needed are usually available to guide administration.
Measurement of the ACT must continue throughout CPB (usually every 30 mins) to ensure that anticoagulation at an appropriate level is maintained.
In CPB, the blood is exposed to a number of prothrombotic and proinflammatory (which is in itself prothrombotic) triggers.
This includes:
- Exposure to the material of the bypass circuit itself.
- Exposure to tissue factor in the wound
- Exposure to air
These all act to initiate the haemostatic pathways, through platelet action and the enzymes of the coagulation pathway.
This leads the the development of fibrin strands, with consumption of platelets, clotting factors and fibrinogen.
As such, anticoagulation is an essential component of CPB.
There are also a number of adverse effects on the haemostatic system as a consequence of of CPB which are discussed later.
Anticoagulation is usually achieved through the administration of unfractionated heparin.
This must be done prior to initiation of CPB.
The target is an activated clotting time (ACT) of 4 times the normal range (normal is around 120s, so a target of 480s is generally used).
A dose of 300-400 units/kg is generally used as a guide for the loading dose needed for anticoagulation to this level.
However, there is a degree of variability in response between patients.
Point of care tests which more accurately measure the dose needed are usually available to guide administration.
Measurement of the ACT must continue throughout CPB (usually every 30 mins) to ensure that anticoagulation at an appropriate level is maintained.
The Process of CPB
The steps involved in CPB are as follows:
The components in more detail:
- Preparation of the circuit
- Heparinisation
- Insertion of the aortic cannula
- Insertion of the right atrial/venous cannula
- Commencement of CPB
- Cross clamping of aorta
- Cardioplegia
- Surgical procedure
- Weaning of CPB
- Removal of venous cannula
- Removal of aortic cannula
- Reversal of heparinisation
The components in more detail:
This is quite a good overview video of CPB:
https://www.youtube.com/watch?v=qFXxzBZt5L
https://www.youtube.com/watch?v=qFXxzBZt5L
Preparation of the circuit
The circuit is primed with fluid before initiation of bypass.
This is because it is impossible to have a break in the circuit with entrainment of air or loss of circulating volume into the circuit.
The choice of fluid is based on calculations of the physical and physiological impact it will have on the blood and circulation.
Crystalloid is used to provide some haemodilution, reducing blood viscosity and thus improving flow dynamics and reducing circuit pressures.
However, over-haemodilution is not desired due to the reduction on oxygen carrying capacity of the blood.
Colloid may also be used to provide some oncotic pressure that is reduced with haemodilution.
The primining solution may also include:
The resulting haemodilution that would occur when this volume is added to the circulation is calculated.
Typically, a haematocrit above 20% is desired in adults.
The circuit is primed with fluid before initiation of bypass.
This is because it is impossible to have a break in the circuit with entrainment of air or loss of circulating volume into the circuit.
The choice of fluid is based on calculations of the physical and physiological impact it will have on the blood and circulation.
Crystalloid is used to provide some haemodilution, reducing blood viscosity and thus improving flow dynamics and reducing circuit pressures.
However, over-haemodilution is not desired due to the reduction on oxygen carrying capacity of the blood.
Colloid may also be used to provide some oncotic pressure that is reduced with haemodilution.
The primining solution may also include:
- Bicarbonate - to act as a buffer
- Heparin
- Blood - if excess haemodilution would be caused
The resulting haemodilution that would occur when this volume is added to the circulation is calculated.
Typically, a haematocrit above 20% is desired in adults.
Heparinisation
This is required prior to the initiation of bypass, as discussed above.
This is required prior to the initiation of bypass, as discussed above.
Insertion of the aortic cannula
The ascending aorta is cannulated by the surgeon.
A degree of hypotension is required at this point to reduce the risk of aortic dissection (due to the pressurised blood entering the wall).
The systolic pressure target may be guided by the surgeon.
A SBP of 80-100 mmHg is sometimes used.
Once the aorta is cannulated, the cannula can be used for the rapid infusion of fluids by the perfusionist.
The size of the aortic cannula is less important than the venous one, as the blood is returned at relatively high pressures (160 - 180 mmHg).
However, if the calibre is particularly narrow, there is increased chance of turbulent flow developing (Reynold’s number).
Alternative sites of cannula insertion (for example if a sternotomy is not being performed) include: femoral artery axillary artery, LV apex.
The ascending aorta is cannulated by the surgeon.
A degree of hypotension is required at this point to reduce the risk of aortic dissection (due to the pressurised blood entering the wall).
The systolic pressure target may be guided by the surgeon.
A SBP of 80-100 mmHg is sometimes used.
Once the aorta is cannulated, the cannula can be used for the rapid infusion of fluids by the perfusionist.
The size of the aortic cannula is less important than the venous one, as the blood is returned at relatively high pressures (160 - 180 mmHg).
However, if the calibre is particularly narrow, there is increased chance of turbulent flow developing (Reynold’s number).
Alternative sites of cannula insertion (for example if a sternotomy is not being performed) include: femoral artery axillary artery, LV apex.
Insertion of the right atrial/venous cannula
The right sided venous cannula is usually inserted into the right atrium.
Some surgical procedures e.g. tricuspid valve surgery, may require separate IVC and SVC cannulae.
The blood is drained from the right sided circulation under the influence of gravity.
As there is a minimal driving pressure, the calibre of the the cannula becomes the major determining factor on the flow possible (again see Hagen-Poiseuille).
The right sided venous cannula is usually inserted into the right atrium.
Some surgical procedures e.g. tricuspid valve surgery, may require separate IVC and SVC cannulae.
The blood is drained from the right sided circulation under the influence of gravity.
As there is a minimal driving pressure, the calibre of the the cannula becomes the major determining factor on the flow possible (again see Hagen-Poiseuille).
Commencement of CPB
When all checks have been done and the team is ready, bypass can commence.
The venous arm of the circuit is unclamped and blood is drained from the right sided circulation before being pumped through the circuit.
Initially, the blood enters a reservoir before being filtered, and so the solution that has primed the circuit is what is returned to the patient.
The flow of blood through the circuit, and therefore the patients new ‘cardiac output’, is controlled by the perfusionist.
The flow rate needed is calculated by the equation:
CPB flow = Cardiac Index x Body Surface Area
As you can see, this will provide a suitable cardiac output for the patient’s height and weight.
The cardiac index is usually 2.4 L/m^2/min, though up to 2.8 depending on the patient’s age, and lower at lower temperatures.
The flow produced by the CPB is continuous rather than pulsatile like the normal arterial waveform, so will display as a relatively flat line on the arterial line monitor.
The perfusionist will monitor a number of values continuously whilst the patient is on CPB, such as:
Ventilation will stop once CPB is established.
Anaesthesia, in the form of a volatile agent, can be administered by the perfusionist.
This is administered in a similar way to normal volatile agent, but using a blood-gas interface in the CPB circuit rather than in the lungs.
As well as blood flow, arterial blood pressure is also required to perfuse vital organs.
Vasopressor agent are administered by the perfusionist in a similar way to under normal anaesthesia conditions, manipulating the systemic vascular resistance to achieve the desired pressure.
When all checks have been done and the team is ready, bypass can commence.
The venous arm of the circuit is unclamped and blood is drained from the right sided circulation before being pumped through the circuit.
Initially, the blood enters a reservoir before being filtered, and so the solution that has primed the circuit is what is returned to the patient.
The flow of blood through the circuit, and therefore the patients new ‘cardiac output’, is controlled by the perfusionist.
The flow rate needed is calculated by the equation:
CPB flow = Cardiac Index x Body Surface Area
As you can see, this will provide a suitable cardiac output for the patient’s height and weight.
The cardiac index is usually 2.4 L/m^2/min, though up to 2.8 depending on the patient’s age, and lower at lower temperatures.
The flow produced by the CPB is continuous rather than pulsatile like the normal arterial waveform, so will display as a relatively flat line on the arterial line monitor.
The perfusionist will monitor a number of values continuously whilst the patient is on CPB, such as:
- Arterial pO2
- Arterial pCO2
- Arterial SpO2
- Venous SpO2
- Circuit pressure
- Haematocrit
- Electrolytes
Ventilation will stop once CPB is established.
Anaesthesia, in the form of a volatile agent, can be administered by the perfusionist.
This is administered in a similar way to normal volatile agent, but using a blood-gas interface in the CPB circuit rather than in the lungs.
As well as blood flow, arterial blood pressure is also required to perfuse vital organs.
Vasopressor agent are administered by the perfusionist in a similar way to under normal anaesthesia conditions, manipulating the systemic vascular resistance to achieve the desired pressure.
Cross clamping of aorta
Once CPB is established, the aorta can be cross-clamped.
All of the major respiratory and cardiac functions are now being performed by the CPB machine.
It is also important to note that now is the time that blood flow to the myocardium will stop, and thus it is from here that the ‘clock starts ticking’.
Once CPB is established, the aorta can be cross-clamped.
All of the major respiratory and cardiac functions are now being performed by the CPB machine.
It is also important to note that now is the time that blood flow to the myocardium will stop, and thus it is from here that the ‘clock starts ticking’.
Cardioplegia
Cardioplegia (or other approaches to myocardial protection) can now be instigated.
This is discussed above.
Cardioplegia (or other approaches to myocardial protection) can now be instigated.
This is discussed above.
Surgical procedure
The surgeon now has access to a still and (relatively) bloodless field to operate in.
Bleeding during this can be suctioned up and returned to the venous reservoir on the CPB machine and thus returned to the circulation.
Of notes, any suctioning of blood after reversal of heparinisation has to go to a separate cell-salvage circuit to avoid it causing clotting in the CPB.
The patient will be off CPB at this time, but there is often a degree of blood left in the circuit that is gradually returned.
During the surgery, there may still be blood entering the heart e.g. from collateral circulation sources, cardioplegia.
This would cause overdistension and permanent damage to the heart if allowed to persist, so ‘venting’ of the left ventricle is also often needed.
The surgeon now has access to a still and (relatively) bloodless field to operate in.
Bleeding during this can be suctioned up and returned to the venous reservoir on the CPB machine and thus returned to the circulation.
Of notes, any suctioning of blood after reversal of heparinisation has to go to a separate cell-salvage circuit to avoid it causing clotting in the CPB.
The patient will be off CPB at this time, but there is often a degree of blood left in the circuit that is gradually returned.
During the surgery, there may still be blood entering the heart e.g. from collateral circulation sources, cardioplegia.
This would cause overdistension and permanent damage to the heart if allowed to persist, so ‘venting’ of the left ventricle is also often needed.
Weaning off CPB
Once the surgical procedure is complete, the process of weaning from the CPB machine can start.
Near normal physiological parameters should be present, including rewarming of the patient.
Ventilation must be recommenced by the anaesthetist.
Release of the aortic cross clamp is the first step and will have allow blood to re-enter the coronary circulation, washing out the cardioplegia solution, and thus allowing the heart to resume its electrical activity.
It can take some time for this occur and the heart transitions through quite abnormal electrical conduction patterns.
If the heart remains relatively slow in rate at this time, pacing wires are frequently inserted to pace the heart to a higher rate of 80-90 bpm to optimise weaning.
The venous tube is then gradually clamped.
This starts to allow blood to re-enter the heart, triggering mechanical cardiac activity.
The heart will slowly start to contribute to the overall cardiac output.
Once a good degree of function has been established, the venous tubing can be clamped entirely.
Blood that remains in the circuit can be administered as boluses through the aortic cannula.
This is done cautiously to minimise cardiovascular disturbance.
This is a challenging part of the whole procedure, and requires the presence of an experienced cardiac anaesthetist.
There is often simultaneous imaging of the heart with transoesophageal echo, to identify any potential problems and guide treatment (e.g. there may be a need for inotropes).
Once the surgical procedure is complete, the process of weaning from the CPB machine can start.
Near normal physiological parameters should be present, including rewarming of the patient.
Ventilation must be recommenced by the anaesthetist.
Release of the aortic cross clamp is the first step and will have allow blood to re-enter the coronary circulation, washing out the cardioplegia solution, and thus allowing the heart to resume its electrical activity.
It can take some time for this occur and the heart transitions through quite abnormal electrical conduction patterns.
If the heart remains relatively slow in rate at this time, pacing wires are frequently inserted to pace the heart to a higher rate of 80-90 bpm to optimise weaning.
The venous tube is then gradually clamped.
This starts to allow blood to re-enter the heart, triggering mechanical cardiac activity.
The heart will slowly start to contribute to the overall cardiac output.
Once a good degree of function has been established, the venous tubing can be clamped entirely.
Blood that remains in the circuit can be administered as boluses through the aortic cannula.
This is done cautiously to minimise cardiovascular disturbance.
This is a challenging part of the whole procedure, and requires the presence of an experienced cardiac anaesthetist.
There is often simultaneous imaging of the heart with transoesophageal echo, to identify any potential problems and guide treatment (e.g. there may be a need for inotropes).
Removal of venous cannula
Once the team are happy that there is adequate cardiovascular stability, the venous cannula is removed and the puncture closed.
Once the team are happy that there is adequate cardiovascular stability, the venous cannula is removed and the puncture closed.
Removal of aortic cannula
Blood from the CPB circuit continues to be cautiously returned via the aortic cannula as tolerated by the patient's cardiovascular system.
Ideally, all of the patient’s blood is returned to minimise the haemoglobin drop.
Once this is complete, the aortic cannula can be removed.
As with insertion, a relative degree of hypotension is best to reduce the risk of blood being driven into the aortic wall and leading to dissection.
Blood from the CPB circuit continues to be cautiously returned via the aortic cannula as tolerated by the patient's cardiovascular system.
Ideally, all of the patient’s blood is returned to minimise the haemoglobin drop.
Once this is complete, the aortic cannula can be removed.
As with insertion, a relative degree of hypotension is best to reduce the risk of blood being driven into the aortic wall and leading to dissection.
Reversal of heparinisation
The heparinisation can now be reversed, though can be started once the patient is stable off CPB.
This is done with protamine.
This must be given slowly, as there can be notable cardiovascular disturbance from it.
The heparinisation can now be reversed, though can be started once the patient is stable off CPB.
This is done with protamine.
This must be given slowly, as there can be notable cardiovascular disturbance from it.
Links & References
- Machin, D. Allsager, C. Principles of cardiopulmonary bypass. CEACCP. 2006. 6(5): 176-181
- Hughes, D. Cardiopulmonary bypass. Blackpool, Fylde and Wyre Hospitals. 2003.
- Allman, K. Wilson, I (eds). Cardiopulmonary bypass, in: Oxford Handbook of Anaesthesia (3rd ed). Oxford University Press. 2011.